An autopsy study of the spectrum of severe COVID-19 in children: From SARS to different phenotypes of MIS-C
- PMID: 33937731
- PMCID: PMC8072136
- DOI: 10.1016/j.eclinm.2021.100850
An autopsy study of the spectrum of severe COVID-19 in children: From SARS to different phenotypes of MIS-C
Abstract
Background: COVID-19 in children is usually mild or asymptomatic, but severe and fatal paediatric cases have been described. The pathology of COVID-19 in children is not known; the proposed pathogenesis for severe cases includes immune-mediated mechanisms or the direct effect of SARS-CoV-2 on tissues. We describe the autopsy findings in five cases of paediatric COVID-19 and provide mechanistic insight into the mechanisms involved in the pathogenesis of the disease.
Methods: Children and adolescents who died with COVID-19 between March 18 and August 15, 2020 were autopsied with a minimally invasive method. Tissue samples from all vital organs were analysed by histology, electron microscopy (EM), reverse-transcription polymerase chain reaction (RT-PCR) and immunohistochemistry (IHC).
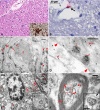
Findings: Five patients were included, one male and four female, aged 7 months to 15 years. Two patients had severe diseases before SARS-CoV-2 infection: adrenal carcinoma and Edwards syndrome. Three patients were previously healthy and had multisystem inflammatory syndrome in children (MIS-C) with distinct clinical presentations: myocarditis, colitis, and acute encephalopathy with status epilepticus. Autopsy findings varied amongst patients and included mild to severe COVID-19 pneumonia, pulmonary microthrombosis, cerebral oedema with reactive gliosis, myocarditis, intestinal inflammation, and haemophagocytosis. SARS-CoV-2 was detected in all patients in lungs, heart and kidneys by at least one method (RT-PCR, IHC or EM), and in endothelial cells from heart and brain in two patients with MIS-C (IHC). In addition, we show for the first time the presence of SARS-CoV-2 in the brain tissue of a child with MIS-C with acute encephalopathy, and in the intestinal tissue of a child with acute colitis. Interpretation: SARS-CoV-2 can infect several cell and tissue types in paediatric patients, and the target organ for the clinical manifestation varies amongst individuals. Two major patterns of severe COVID-19 were observed: a primarily pulmonary disease, with severe acute respiratory disease and diffuse alveolar damage, or a multisystem inflammatory syndrome with the involvement of several organs. The presence of SARS-CoV-2 in several organs, associated with cellular ultrastructural changes, reinforces the hypothesis that a direct effect of SARS-CoV-2 on tissues is involved in the pathogenesis of MIS-C.
Funding: Fundação de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Bill and Melinda Gates Foundation.
Keywords: Autopsy; Children; Covid-19; MIS-C, multisystem inflammatory syndrome in children; Minimally invasive autopsy; Pathology; Sars-cov-2.
© 2021 The Author(s).
Conflict of interest statement
We declare no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

