SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy
- PMID: 33937733
- PMCID: PMC8079668
- DOI: 10.1016/j.eclinm.2021.100861
SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy
Abstract
Background: Reinfection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been documented, raising public health concerns. SARS-CoV-2 reinfections were assessed in a cohort of antibody-positive persons in Qatar.
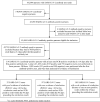
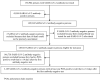
Methods: All SARS-CoV-2 antibody-positive persons from April 16 to December 31, 2020 with a PCR-positive swab ≥14 days after the first-positive antibody test were investigated for evidence of reinfection. Viral genome sequencing was conducted for paired viral specimens to confirm reinfection. Incidence of reinfection was compared to incidence of infection in the complement cohort of those who were antibody-negative.
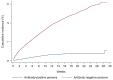
Findings: Among 43,044 antibody-positive persons who were followed for a median of 16.3 weeks (range: 0-34.6), 314 individuals (0.7%) had at least one PCR positive swab ≥14 days after the first-positive antibody test. Of these individuals, 129 (41.1%) had supporting epidemiological evidence for reinfection. Reinfection was next investigated using viral genome sequencing. Applying the viral-genome-sequencing confirmation rate, the incidence rate of reinfection was estimated at 0.66 per 10,000 person-weeks (95% CI: 0.56-0.78). Incidence rate of reinfection versus month of follow-up did not show any evidence of waning of immunity for over seven months of follow-up. Meanwhile, in the complement cohort of 149,923 antibody-negative persons followed for a median of 17.0 weeks (range: 0-45.6), incidence rate of infection was estimated at 13.69 per 10,000 person-weeks (95% CI: 13.22-14.14). Efficacy of natural infection against reinfection was estimated at 95.2% (95% CI: 94.1-96.0%). Reinfections were less severe than primary infections. Only one reinfection was severe, two were moderate, and none were critical or fatal. Most reinfections (66.7%) were diagnosed incidentally through random or routine testing, or through contact tracing.
Interpretation: Reinfection is rare in the young and international population of Qatar. Natural infection appears to elicit strong protection against reinfection with an efficacy ~95% for at least seven months.
Funding: Biomedical Research Program, the Biostatistics, Epidemiology, and Biomathematics Research Core, and the Genomics Core, all at Weill Cornell Medicine-Qatar, the Ministry of Public Health, Hamad Medical Corporation, and the Qatar Genome Programme.
Keywords: Epidemiology; Genetics; Immunity; Reinfection; SARS-CoV-2.
© 2021 The Author(s).
Conflict of interest statement
We declare no competing interests.
Figures


References
-
- World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re.... Accessed on March 14, 2020.
-
- De Walque D, Friedman J, Gatti RV, Mattoo A. How two tests can help contain COVID-19 and revive the economy. Available from: http://documents.worldbank.org/curated/en/766471586360658318/pdf/How-Two.... Accessed on April 16, 2020. Research & Policy Briefs, World Bank Malaysia Hub 2020.
-
- Kaplan J, Frias L, McFall-Johnsen M. A third of the global population is on coronavirus lockdown. Available from: https://www.businessinsider.com.au/countries-on-lockdown-coronavirus-ita.... Accessd on: April 25, 2020. Business Insider Australia 2020.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

