Put a ring on it: application of small aliphatic rings in medicinal chemistry
- PMID: 33937776
- PMCID: PMC8083977
- DOI: 10.1039/d0md00370k
Put a ring on it: application of small aliphatic rings in medicinal chemistry
Abstract
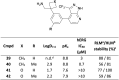
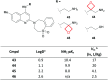
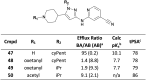
Aliphatic three- and four-membered rings including cyclopropanes, cyclobutanes, oxetanes, azetidines and bicyclo[1.1.1]pentanes have been increasingly exploited in medicinal chemistry for their beneficial physicochemical properties and applications as functional group bioisosteres. This review provides a historical perspective and comparative up to date overview of commonly applied small rings, exemplifying key principles with recent literature examples. In addition to describing the merits and advantages of each ring system, potential hazards and liabilities are also illustrated and explained, including any significant chemical or metabolic stability and toxicity risks.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All authors are employees of AstraZeneca.
Figures
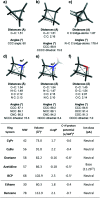

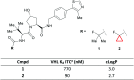
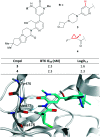
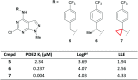
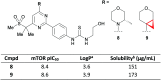
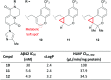

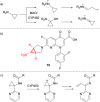

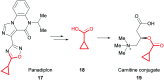
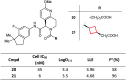

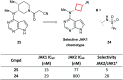
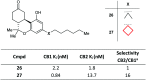
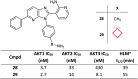


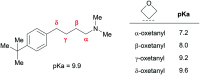

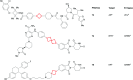

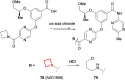
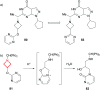
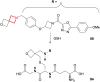

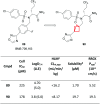

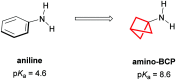
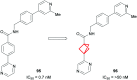
References
-
- Lovering F. MedChemComm. 2013;4:515–519. doi: 10.1039/C2MD20347B. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

