Endohedral Mixed Aggregates: Sodium Alkoxide Cages with Organic or Inorganic Central Anions and Variable Hull
- PMID: 33938049
- PMCID: PMC8453731
- DOI: 10.1002/chem.202100912
Endohedral Mixed Aggregates: Sodium Alkoxide Cages with Organic or Inorganic Central Anions and Variable Hull
Abstract
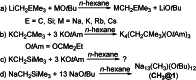
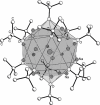
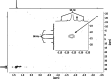
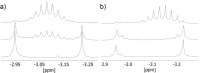
Alkali metal alkoxides are widely used in chemistry due to their Brønsted basic and nucleophilic properties. Potassium alkoxides assist alkyllithium in the metalation of hydrocarbons in Lochmann-Schlosser-bases. Both compounds form mixed aggregates, which enhance the thermal stability, solubility, and the basic reactivity of these mixtures. A very unusual spherical mixed alkoxy aggregate was discovered by Grützmacher et al., where a central dihydrogen phosphide anion is surrounded by a highly dynamic shell of thirteen sodium atoms and a hull of twelve tert-butoxide groups. This structural motif can be reproduced by a reaction of trimethylsilyl compounds of methane, halogens, or pseudo-halogens with excess sodium tert-butoxide. A nucleophilic substitution releases the corresponding anion, which is then encapsulated by the sodium alkoxide units. The compounds are soluble in hydrocarbon solvents, enabling studies of solutions by high-resolution NMR spectroscopy and IR/Raman studies of the crystalline materials.
Keywords: aggregation; alkoxide; cage compounds; isotopic labeling; sodium.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Combining Neopentyllithium with Potassium tert-Butoxide: Formation of an Alkane-Soluble Lochmann-Schlosser Superbase.Angew Chem Int Ed Engl. 2016 Aug 26;55(36):10886-9. doi: 10.1002/anie.201602792. Epub 2016 Jul 8. Angew Chem Int Ed Engl. 2016. PMID: 27392232
-
Towards the Next Generation of Lochmann-Schlosser Superbases: A Potassium Neopentyl/Alkoxy Aggregate used in the Tetra-Functionalization of Ferrocene.Chemistry. 2018 May 28;24(30):7605-7609. doi: 10.1002/chem.201800608. Epub 2018 May 3. Chemistry. 2018. PMID: 29529351
-
Towards Substrate-Reagent Interaction of Lochmann-Schlosser Bases in THF: Bridging THF Hides Potential Reaction Site of a Chiral Superbase.Chemistry. 2022 Dec 9;28(69):e202202660. doi: 10.1002/chem.202202660. Epub 2022 Oct 17. Chemistry. 2022. PMID: 36098179 Free PMC article.
-
Structural Motifs of Alkali Metal Superbases in Non-coordinating Solvents.Chemistry. 2021 Jan 13;27(3):888-904. doi: 10.1002/chem.202002812. Epub 2020 Nov 9. Chemistry. 2021. PMID: 33165981 Free PMC article. Review.
-
Sense or no-sense of the sum parameter for water soluble "adsorbable organic halogens" (AOX) and "absorbed organic halogens" (AOX-S18) for the assessment of organohalogens in sludges and sediments.Chemosphere. 2003 Jul;52(2):371-9. doi: 10.1016/S0045-6535(03)00215-7. Chemosphere. 2003. PMID: 12738259 Review.
Cited by
-
Rapid Access to Isoprenoid Quinones through X@RONa-Catalyzed Redox Chain Reaction.J Am Chem Soc. 2024 Oct 23;146(42):29064-29071. doi: 10.1021/jacs.4c10470. Epub 2024 Oct 9. J Am Chem Soc. 2024. PMID: 39384527 Free PMC article.
References
-
- Østreng E., Sønsteby H. H., Øien S., Nilsen O., Fjellvåg H., Dalton Trans. 2014, 43, 16666–16672. - PubMed
-
- None
-
- Speier J. L., J. Am. Chem. Soc. 1948, 70, 4142–4143; - PubMed
-
- Magnus P., Roy G., Organometallics 1982, 1, 553–559;
-
- Feuer H., Hooz J., The Ether Linkage, John Wiley & Sons, Ltd, Chichester, UK, 1967, p. 445.
LinkOut - more resources
Full Text Sources
Other Literature Sources

