ID1 and ID3 are Negative Regulators of TGFβ2-Induced Ocular Hypertension and Compromised Aqueous Humor Outflow Facility in Mice
- PMID: 33938911
- PMCID: PMC8107646
- DOI: 10.1167/iovs.62.6.3
ID1 and ID3 are Negative Regulators of TGFβ2-Induced Ocular Hypertension and Compromised Aqueous Humor Outflow Facility in Mice
Abstract
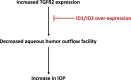
Purpose: In POAG, elevated IOP remains the major risk factor in irreversible vision loss. Increased TGFβ2 expression in POAG aqueous humor and in the trabecular meshwork (TM) amplifies extracellular matrix (ECM) deposition and reduces ECM turnover in the TM, leading to a decreased aqueous humor (AH) outflow facility and increased IOP. Inhibitor of DNA binding proteins (ID1 and ID3) inhibit TGFβ2-induced fibronectin and PAI-1 production in TM cells. We examined the effects of ID1 and ID3 gene expression on TGFβ2-induced ocular hypertension and decreased AH outflow facility in living mouse eyes.
Methods: IOP and AH outflow facility changes were determined using a mouse model of Ad5-hTGFβ2C226S/C288S-induced ocular hypertension. The physiological function of ID1 and ID3 genes were evaluated using Ad5 viral vectors to enhance or knockdown ID1/ID3 gene expression in the TM of BALB/cJ mice. IOP was measured in conscious mice using a Tonolab impact tonometer. AH outflow facilities were determined by constant flow infusion in live mice.
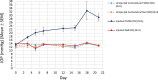
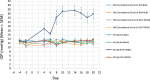
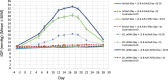
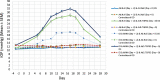
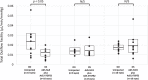
Results: Over-expressing ID1 and ID3 significantly blocked TGFβ2-induced ocular hypertension (P < 0.0001). Although AH outflow facility was significantly decreased in TGFβ2-transduced eyes (P < 0.04), normal outflow facility was preserved in eyes injected concurrently with ID1 or ID3 along with TGFβ2. Knockdown of ID1 or ID3 expression exacerbated TGFβ2-induced ocular hypertension.
Conclusions: Increased expression of ID1 and ID3 suppressed both TGFβ2-elevated IOP and decreased AH outflow facility. ID1 and/or ID3 proteins thus may show promise as future candidates as IOP-lowering targets in POAG.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes.Invest Ophthalmol Vis Sci. 2010 Apr;51(4):2067-76. doi: 10.1167/iovs.09-4567. Epub 2009 Dec 3. Invest Ophthalmol Vis Sci. 2010. PMID: 19959644
-
BMP and Activin Membrane Bound Inhibitor Regulates the Extracellular Matrix in the Trabecular Meshwork.Invest Ophthalmol Vis Sci. 2018 Apr 1;59(5):2154-2166. doi: 10.1167/iovs.17-23282. Invest Ophthalmol Vis Sci. 2018. PMID: 29801150 Free PMC article.
-
Nuclear factor-kappa beta signaling is required for transforming growth factor Beta-2 induced ocular hypertension.Exp Eye Res. 2020 Feb;191:107920. doi: 10.1016/j.exer.2020.107920. Epub 2020 Jan 8. Exp Eye Res. 2020. PMID: 31923415 Free PMC article.
-
Smad3 is necessary for transforming growth factor-beta2 induced ocular hypertension in mice.Exp Eye Res. 2013 Nov;116:419-23. doi: 10.1016/j.exer.2013.10.017. Epub 2013 Oct 31. Exp Eye Res. 2013. PMID: 24184030 Free PMC article. Review.
-
Pressure-induced expression changes in segmental flow regions of the human trabecular meshwork.Exp Eye Res. 2017 May;158:67-72. doi: 10.1016/j.exer.2016.06.009. Epub 2016 Jun 19. Exp Eye Res. 2017. PMID: 27334250 Free PMC article. Review.
Cited by
-
Single-Cell RNA Sequencing Analysis of the Early Postnatal Mouse Lens Epithelium.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):37. doi: 10.1167/iovs.64.13.37. Invest Ophthalmol Vis Sci. 2023. PMID: 37870847 Free PMC article.
-
Regenerative Therapy for Corneal Scarring Disorders.Biomedicines. 2024 Mar 14;12(3):649. doi: 10.3390/biomedicines12030649. Biomedicines. 2024. PMID: 38540264 Free PMC article. Review.
-
CCN2/CTGF tip the balance of growth factors towards TGF-β2 in primary open-angle glaucoma.Front Mol Biosci. 2023 May 11;10:1045411. doi: 10.3389/fmolb.2023.1045411. eCollection 2023. Front Mol Biosci. 2023. PMID: 37251082 Free PMC article.
-
TGFβ2 alters segmental outflow and ECM ultrastructure in the trabecular meshwork.Exp Eye Res. 2025 Jun;255:110377. doi: 10.1016/j.exer.2025.110377. Epub 2025 Apr 10. Exp Eye Res. 2025. PMID: 40216065
-
Inhibition of TGF-β2-Induced Trabecular Meshwork Fibrosis by Pirfenidone.Transl Vis Sci Technol. 2023 Nov 1;12(11):21. doi: 10.1167/tvst.12.11.21. Transl Vis Sci Technol. 2023. PMID: 37975842 Free PMC article.
References
-
- Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017; 390(10108): 2183–2193. - PubMed
-
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130: 429–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

