One-year performance of posterior narrow diameter implants in hyperglycemic and normo-glycemic patients-a pilot study
- PMID: 33939006
- PMCID: PMC8602141
- DOI: 10.1007/s00784-021-03957-x
One-year performance of posterior narrow diameter implants in hyperglycemic and normo-glycemic patients-a pilot study
Abstract
Objectives: The aim of the study was to compare the performance of narrow diameter implants in patients with uncontrolled diabetes mellitus type 2 (T2DM) and normo-glycemic individuals during the first 12 months after implant loading.
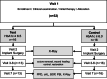
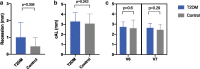
Material and methods: In 16 T2DM patients with HbA1C > 6.5% (test group) and 16 normo-glycemic patients (HbA1C < 6.0%; control group), one to two narrow diameter tissue level implants were placed in the posterior maxilla or mandible. After 3-month lasting integration period, implants were loaded by fixed dentures. The clinical parameters probing depth (PD), bleeding on probing (BOP), attachment loss (CAL), recession and papilla bleeding index (PBI) were assessed manually at loading and after 12 months of function. The paired digital periapical radiographs were analyzed with regard to the change in marginal bone level (MBL) from baseline to 12 months' control. The mean values calculated for both patient groups were statistically analyzed. The technical complications were recorded.
Results: The T2DM group accounted 13 patients due to 3 dropouts. The overall implant survival rate after 12 months was 100%. The differences in means for the clinical parameters and the MBL were statistically non-significant between the T2DM and normo-glycemic patients for the short period of loaded function reported here. No technical complications were recorded.
Conclusions: The study demonstrated an encouraging clinical outcome with narrow diameter implants in patients with uncontrolled T2DM compared to non-diabetics after 12 months post loading. For the short observation period, no biological and technical complications were reported regardless the glycemic status.
Clinical relevance: Patients with HbA1C > 6.5% may benefit from the treatment with narrow diameter implants by avoiding complex surgical interventions with augmentation procedures.
Trial registration: Clinicaltrials.gov : NCT04630691.
Keywords: Marginal bone loss; Narrow diameter implants; SLActive surface; TiZr implants; Type 2 diabetes mellitus.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Restoration of posterior teeth by narrow diameter implants in hyperglycemic and normoglycemic patients - 4-year results of a case-control study.Clin Oral Investig. 2024 Jun 22;28(7):392. doi: 10.1007/s00784-024-05786-0. Clin Oral Investig. 2024. PMID: 38907052 Free PMC article.
-
Clinical and radiographic evaluation of narrow-diameter titanium-zirconium implants in unilateral atrophic mandibular distal extensions: a 1-year pilot study.J Contemp Dent Pract. 2014 Jul 1;15(4):417-22. doi: 10.5005/jp-journals-10024-1555. J Contemp Dent Pract. 2014. PMID: 25576106
-
One-piece zirconia oral implants: one-year results from a prospective case series. 2. Three-unit fixed dental prosthesis (FDP) reconstruction.J Clin Periodontol. 2013 May;40(5):553-62. doi: 10.1111/jcpe.12093. Epub 2013 Mar 18. J Clin Periodontol. 2013. PMID: 23506654
-
Clinical, radiographic and economic evaluation of short-6-mm implants and longer implants combined with osteotome sinus floor elevation in moderately atrophic maxillae: A 3-year randomized clinical trial.J Clin Periodontol. 2021 May;48(5):695-704. doi: 10.1111/jcpe.13444. Epub 2021 Mar 8. J Clin Periodontol. 2021. PMID: 33570787 Review.
-
Influence of implant diameter on implant survival rate and clinical outcomes in the posterior area: a systematic review and meta-analysis.BMC Oral Health. 2023 Apr 21;23(1):235. doi: 10.1186/s12903-023-02962-8. BMC Oral Health. 2023. PMID: 37085829 Free PMC article.
Cited by
-
Implant stability of narrow diameter implants in hyperglycemic patients-A 3-month case-control study.Clin Exp Dent Res. 2022 Aug;8(4):969-975. doi: 10.1002/cre2.587. Epub 2022 May 16. Clin Exp Dent Res. 2022. PMID: 35578391 Free PMC article.
-
Microcirculation and neutrophil-related cytokine concentrations are not altered around narrow diameter implants in T2DM patients during wound healing.Clin Oral Investig. 2023 Mar;27(3):1167-1175. doi: 10.1007/s00784-022-04731-3. Epub 2022 Oct 13. Clin Oral Investig. 2023. PMID: 36229741 Free PMC article.
-
Systemic Diseases and Biological Dental Implant Complications: A Narrative Review.Dent J (Basel). 2022 Dec 29;11(1):10. doi: 10.3390/dj11010010. Dent J (Basel). 2022. PMID: 36661547 Free PMC article. Review.
-
Restoration of posterior teeth by narrow diameter implants in hyperglycemic and normoglycemic patients - 4-year results of a case-control study.Clin Oral Investig. 2024 Jun 22;28(7):392. doi: 10.1007/s00784-024-05786-0. Clin Oral Investig. 2024. PMID: 38907052 Free PMC article.
References
-
- Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016;12:616–622. - PubMed
-
- Alasqah MN, Alrabiah M, Al-Aali KA, Mokeem SA, Binmahfooz AM, ArRejaie AS, Abduljabbar T. Peri-implant soft tissue status and crestal bone levels around adjacent implants placed in patients with and without type-2 diabetes mellitus: 6 years follow-up results. Clin Implant Dent Relat Res. 2018;20:562–568. doi: 10.1111/cid.12617. - DOI - PubMed
-
- Al-Sowygh ZH, Ghani SMA, Sergis K, Vohra F, Akram Z. Peri-implant conditions and levels of advanced glycation end products among patients with different glycemic control. Clin Implant Dent Relat Res. 2018;00:1–7. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

