Senescence-associated β-galactosidase reveals the abundance of senescent CD8+ T cells in aging humans
- PMID: 33939265
- PMCID: PMC8135084
- DOI: 10.1111/acel.13344
Senescence-associated β-galactosidase reveals the abundance of senescent CD8+ T cells in aging humans
Abstract
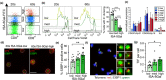
Aging leads to a progressive functional decline of the immune system, rendering the elderly increasingly susceptible to disease and infection. The degree to which immune cell senescence contributes to this decline remains unclear, however, since markers that label immune cells with classical features of cellular senescence accurately and comprehensively have not been identified. Using a second-generation fluorogenic substrate for β-galactosidase and multi-parameter flow cytometry, we demonstrate here that peripheral blood mononuclear cells (PBMCs) isolated from healthy humans increasingly display cells with high senescence-associated β-galactosidase (SA-βGal) activity with advancing donor age. The greatest age-associated increases were observed in CD8+ T-cell populations, in which the fraction of cells with high SA-βGal activity reached average levels of 64% in donors in their 60s. CD8+ T cells with high SA-βGal activity, but not those with low SA-βGal activity, were found to exhibit features of telomere dysfunction-induced senescence and p16-mediated senescence, were impaired in their ability to proliferate, developed in various T-cell differentiation states, and had a gene expression signature consistent with the senescence state previously observed in human fibroblasts. Based on these results, we propose that senescent CD8+ T cells with classical features of cellular senescence accumulate to levels that are significantly higher than previously reported and additionally provide a simple yet robust method for the isolation and characterization of senescent CD8+ T cells with predictive potential for biological age.
Keywords: PBMC; T cells; aging; cellular senescence; immunosenescence; lymphocytes; p16; senescence-associated β-galactosidase; telomere.
© 2021 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures




References
-
- Appay, V. , van Lier, R. A. , Sallusto, F. , & Roederer, M. (2008). Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A, 73, 975–983. - PubMed
-
- Baixauli, F. , Acin‐Perez, R. , Villarroya‐Beltri, C. , Mazzeo, C. , Nunez‐Andrade, N. , Gabande‐Rodriguez, E. , Ledesma, M. D. , Blazquez, A. , Martin, M. A. , Falcon‐Perez, J. M. , Redondo, J. M. , Enriquez, J. A. , & Mittelbrunn, M. (2015). Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metabolism, 22, 485–498. - PMC - PubMed
-
- Baker, D. J. , Childs, B. G. , Durik, M. , Wijers, M. E. , Sieben, C. J. , Zhong, J. , Saltness, R. A. , Jeganathan, K. B. , Verzosa, G. C. , Pezeshki, A. , Khazaie, K. , Miller, J. D. , & van Deursen, J. M. (2016). Naturally occurring p16(Ink4a)‐positive cells shorten healthy lifespan. Nature, 530, 184–189. - PMC - PubMed
-
- Ben‐Porath, I. , & Weinberg, R. A. (2005). The signals and pathways activating cellular senescence. International Journal of Biochemistry & Cell Biology, 37, 961–976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

