Dynamic patterns of correlated activity in the prefrontal cortex encode information about social behavior
- PMID: 33939689
- PMCID: PMC8118626
- DOI: 10.1371/journal.pbio.3001235
Dynamic patterns of correlated activity in the prefrontal cortex encode information about social behavior
Abstract
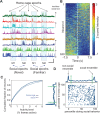
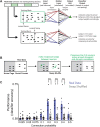
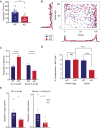
New technologies make it possible to measure activity from many neurons simultaneously. One approach is to analyze simultaneously recorded neurons individually, then group together neurons which increase their activity during similar behaviors into an "ensemble." However, this notion of an ensemble ignores the ability of neurons to act collectively and encode and transmit information in ways that are not reflected by their individual activity levels. We used microendoscopic GCaMP imaging to measure prefrontal activity while mice were either alone or engaged in social interaction. We developed an approach that combines a neural network classifier and surrogate (shuffled) datasets to characterize how neurons synergistically transmit information about social behavior. Notably, unlike optimal linear classifiers, a neural network classifier with a single linear hidden layer can discriminate network states which differ solely in patterns of coactivity, and not in the activity levels of individual neurons. Using this approach, we found that surrogate datasets which preserve behaviorally specific patterns of coactivity (correlations) outperform those which preserve behaviorally driven changes in activity levels but not correlated activity. Thus, social behavior elicits increases in correlated activity that are not explained simply by the activity levels of the underlying neurons, and prefrontal neurons act collectively to transmit information about socialization via these correlations. Notably, this ability of correlated activity to enhance the information transmitted by neuronal ensembles is diminished in mice lacking the autism-associated gene Shank3. These results show that synergy is an important concept for the coding of social behavior which can be disrupted in disease states, reveal a specific mechanism underlying this synergy (social behavior increases correlated activity within specific ensembles), and outline methods for studying how neurons within an ensemble can work together to encode information.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Reduced sociability and social agency encoding in adult Shank3-mutant mice are restored through gene re-expression in real time.Nat Neurosci. 2021 Sep;24(9):1243-1255. doi: 10.1038/s41593-021-00888-4. Epub 2021 Jul 12. Nat Neurosci. 2021. PMID: 34253921 Free PMC article.
-
Neural activity in prefrontal cortex during copying geometrical shapes. II. Decoding shape segments from neural ensembles.Exp Brain Res. 2003 May;150(2):142-53. doi: 10.1007/s00221-003-1417-5. Epub 2003 Apr 1. Exp Brain Res. 2003. PMID: 12669171
-
Distinct and Dynamic ON and OFF Neural Ensembles in the Prefrontal Cortex Code Social Exploration.Neuron. 2018 Nov 7;100(3):700-714.e9. doi: 10.1016/j.neuron.2018.08.043. Epub 2018 Sep 27. Neuron. 2018. PMID: 30269987 Free PMC article.
-
Prefrontal cortex and working memory processes.Neuroscience. 2006 Apr 28;139(1):251-61. doi: 10.1016/j.neuroscience.2005.07.003. Epub 2005 Dec 1. Neuroscience. 2006. PMID: 16325345 Review.
-
Task set and prefrontal cortex.Annu Rev Neurosci. 2008;31:219-45. doi: 10.1146/annurev.neuro.31.060407.125642. Annu Rev Neurosci. 2008. PMID: 18558854 Review.
Cited by
-
A cell-type-specific epigenetic mechanism encodes social investigatory behavior via Lrhcn1 and Hcn1.Sci Adv. 2025 Jun 6;11(23):eadt5400. doi: 10.1126/sciadv.adt5400. Epub 2025 Jun 4. Sci Adv. 2025. PMID: 40465726 Free PMC article.
-
Prefrontal Regulation of Social Behavior and Related Deficits: Insights From Rodent Studies.Biol Psychiatry. 2024 Jul 15;96(2):85-94. doi: 10.1016/j.biopsych.2024.03.008. Epub 2024 Mar 13. Biol Psychiatry. 2024. PMID: 38490368 Free PMC article. Review.
-
Neural manifold analysis of brain circuit dynamics in health and disease.J Comput Neurosci. 2023 Feb;51(1):1-21. doi: 10.1007/s10827-022-00839-3. Epub 2022 Dec 16. J Comput Neurosci. 2023. PMID: 36522604 Free PMC article. Review.
-
Persistent cortical excitatory neuron dysregulation in adult Chd8 haploinsufficient mice.bioRxiv [Preprint]. 2025 Jul 19:2025.06.04.657776. doi: 10.1101/2025.06.04.657776. bioRxiv. 2025. PMID: 40501938 Free PMC article. Preprint.
-
The role of the prefrontal cortex in social interactions of animal models and the implications for autism spectrum disorder.Front Psychiatry. 2023 Jun 20;14:1205199. doi: 10.3389/fpsyt.2023.1205199. eCollection 2023. Front Psychiatry. 2023. PMID: 37409155 Free PMC article. Review.
References
-
- Liang B, Zhang L, Barbera G, Fang W, Zhang J, Chen X, et al.. Distinct and Dynamic ON and OFF Neural Ensembles in the Prefrontal Cortex Code Social Exploration. Neuron [Internet]. 2018. November 7 [cited 2019 Mar 30];100(3):700–14.e9. Available from: https://www.sciencedirect.com/science/article/pii/S0896627318307724?via%... 10.1016/j.neuron.2018.08.043 - DOI - PMC - PubMed
-
- deCharms RC, Merzenich MM. Primary cortical representation of sounds by the coordination of action-potential timing. Nature [Internet]. 1996. June 13 [cited 2019 Aug 25];381(6583):610–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8637597 10.1038/381610a0 - DOI - PubMed
-
- Hebb DO. The organization of behavior: A neuropsychological theory. New York: Wiley; 1949.
-
- Buzsáki G. Neural Syntax: Cell Assemblies, Synapsembles, and Readers. Neuron [Internet]. 2010. November 4 [cited 2019 Mar 30];68(3):362–85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21040841 10.1016/j.neuron.2010.09.023 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

