Small subpopulations of β-cells do not drive islet oscillatory [Ca2+] dynamics via gap junction communication
- PMID: 33939712
- PMCID: PMC8118513
- DOI: 10.1371/journal.pcbi.1008948
Small subpopulations of β-cells do not drive islet oscillatory [Ca2+] dynamics via gap junction communication
Abstract
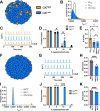
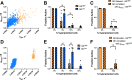
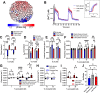
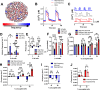
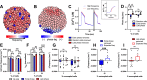
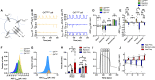
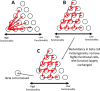
The islets of Langerhans exist as multicellular networks that regulate blood glucose levels. The majority of cells in the islet are excitable, insulin-producing β-cells that are electrically coupled via gap junction channels. β-cells are known to display heterogeneous functionality. However, due to gap junction coupling, β-cells show coordinated [Ca2+] oscillations when stimulated with glucose, and global quiescence when unstimulated. Small subpopulations of highly functional β-cells have been suggested to control [Ca2+] dynamics across the islet. When these populations were targeted by optogenetic silencing or photoablation, [Ca2+] dynamics across the islet were largely disrupted. In this study, we investigated the theoretical basis of these experiments and how small populations can disproportionality control islet [Ca2+] dynamics. Using a multicellular islet model, we generated normal, skewed or bimodal distributions of β-cell heterogeneity. We examined how islet [Ca2+] dynamics were disrupted when cells were targeted via hyperpolarization or populations were removed; to mimic optogenetic silencing or photoablation, respectively. Targeted cell populations were chosen based on characteristics linked to functional subpopulation, including metabolic rate of glucose oxidation or [Ca2+] oscillation frequency. Islets were susceptible to marked suppression of [Ca2+] when ~10% of cells with high metabolic activity were hyperpolarized; where hyperpolarizing cells with normal metabolic activity had little effect. However, when highly metabolic cells were removed from the model, [Ca2+] oscillations remained. Similarly, when ~10% of cells with either the highest frequency or earliest elevations in [Ca2+] were removed from the islet, the [Ca2+] oscillation frequency remained largely unchanged. Overall, these results indicate small populations of β-cells with either increased metabolic activity or increased frequency are unable to disproportionately control islet-wide [Ca2+] via gap junction coupling. Therefore, we need to reconsider the physiological basis for such small β-cell populations or the mechanism by which they may be acting to control normal islet function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
β-cell intrinsic dynamics rather than gap junction structure dictates subpopulations in the islet functional network.Elife. 2023 Nov 29;12:e83147. doi: 10.7554/eLife.83147. Elife. 2023. PMID: 38018905 Free PMC article.
-
Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet.J Physiol. 2011 Nov 15;589(Pt 22):5453-66. doi: 10.1113/jphysiol.2011.218909. Epub 2011 Sep 19. J Physiol. 2011. PMID: 21930600 Free PMC article.
-
Beta-cell hubs maintain Ca2+ oscillations in human and mouse islet simulations.Islets. 2018;10(4):151-167. doi: 10.1080/19382014.2018.1493316. Islets. 2018. PMID: 30142036 Free PMC article.
-
Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics.Trends Endocrinol Metab. 2014 Aug;25(8):399-406. doi: 10.1016/j.tem.2014.02.005. Epub 2014 Mar 26. Trends Endocrinol Metab. 2014. PMID: 24679927 Free PMC article. Review.
-
Connexin 36, a key element in pancreatic beta cell function.Neuropharmacology. 2013 Dec;75:557-66. doi: 10.1016/j.neuropharm.2013.08.015. Epub 2013 Aug 23. Neuropharmacology. 2013. PMID: 23973309 Review.
Cited by
-
The physiological role of β-cell heterogeneity in pancreatic islet function.Nat Rev Endocrinol. 2022 Jan;18(1):9-22. doi: 10.1038/s41574-021-00568-0. Epub 2021 Oct 19. Nat Rev Endocrinol. 2022. PMID: 34667280 Free PMC article. Review.
-
Importance of Both Imprinted Genes and Functional Heterogeneity in Pancreatic Beta Cells: Is There a Link?Int J Mol Sci. 2021 Jan 20;22(3):1000. doi: 10.3390/ijms22031000. Int J Mol Sci. 2021. PMID: 33498234 Free PMC article. Review.
-
Deconstructing the integrated oscillator model for pancreatic β-cells.Math Biosci. 2023 Nov;365:109085. doi: 10.1016/j.mbs.2023.109085. Epub 2023 Oct 4. Math Biosci. 2023. PMID: 37802364 Free PMC article.
-
β-cell intrinsic dynamics rather than gap junction structure dictates subpopulations in the islet functional network.Elife. 2023 Nov 29;12:e83147. doi: 10.7554/eLife.83147. Elife. 2023. PMID: 38018905 Free PMC article.
-
DiabetesOmic: A comprehensive multi-omics diabetes database.Comput Struct Biotechnol J. 2025 May 9;27:2147-2154. doi: 10.1016/j.csbj.2025.05.008. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40502930 Free PMC article.
References
-
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

