Observational cohort study of the effect of a single lubricant exposure during transvaginal ultrasound on cell-shedding from the vaginal epithelium
- PMID: 33939727
- PMCID: PMC8092793
- DOI: 10.1371/journal.pone.0250153
Observational cohort study of the effect of a single lubricant exposure during transvaginal ultrasound on cell-shedding from the vaginal epithelium
Abstract
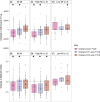
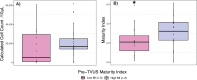
The outer layers of the vaginal epithelium (VE) are important because they accumulate glycogen which, under optimal conditions, Lactobacillus spp. consume to grow and acidify the vaginal microenvironment with lactic acid. We hypothesized that exposure to lubricant, for example in the conduct of a transvaginal ultrasound (TVUS), may contribute to the shedding of mature epithelial cells, exposing immature cells. Cervicovaginal fluid (CVF) was sampled at four time points by menstrual cup (Softdisc™) from 50 women referred for TVUS, during which a controlled volume of lubricant was applied to the TVUS wand. Samples were collected (1) immediately before TVUS and (2) 6-12 hours, (3) within one week, and (4) two weeks after TVUS. Clinical vaginal lubricants are similar to commercial lubricants, and often have a high osmolality or pH, and contain bactericides such as methylparaben and propylparaben. The number and maturity of epithelial cells in each CVF sample were measured by quantitative and differential fluorimetry (maturity index, MI). Comparisons of cell-counts and maturity were made by paired Wilcoxon signed-rank tests. Among women with a high pre-TVUS MI (> 3), there was a decrease in median cell-count and mean MI in the sample collected 6-12 hours after TVUS (p<0.001, n = 26 and p < 0.001, n = 26, respectively). For these women, cell-count and MI remained lower in the sample collected within the subsequent week (p<0.001, n = 29 and p<0.01, n = 29, respectively), and MI remained lower in the sample collected within two weeks of TVUS (p<0.01, n = 25), compared to the pre-TVUS sample. Among participants with a low pre-TVUS MI (< 3), cell-count was higher in the sample collected within two weeks of TVUS compared to the pre-TVUS sample (p = 0.03, n = 15), but no significant changes in MI were observed. Results were similar when restricted to reproductive-age women. This preliminary data indicates hypertonic vaginal lubricants may increase vaginal epithelial cell shedding.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: J.R. is co-founder of LUCA Biologics, a biotechnology company focusing on translating microbiome research into live biotherapeutics drugs for women’s health. All other authors declare that they have no competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88(2):185–94. 10.1016/j.jri.2011.01.005 - DOI - PMC - PubMed
-
- Cruickshank R, Sharman A. The Biology of the Vagina in the Human Subject. BJOG: An International Journal of Obstetrics & Gynaecology. 1934;41(2):208–26.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

