Cerebral dopamine neurotrophic factor reduces α-synuclein aggregation and propagation and alleviates behavioral alterations in vivo
- PMID: 33940158
- PMCID: PMC8417450
- DOI: 10.1016/j.ymthe.2021.04.035
Cerebral dopamine neurotrophic factor reduces α-synuclein aggregation and propagation and alleviates behavioral alterations in vivo
Abstract
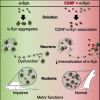
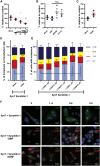
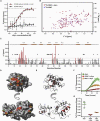
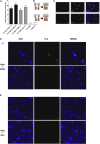
A molecular hallmark in Parkinson's disease (PD) pathogenesis are α-synuclein aggregates. Cerebral dopamine neurotrophic factor (CDNF) is an atypical growth factor that is mostly resident in the endoplasmic reticulum but exerts its effects both intracellularly and extracellularly. One of the beneficial effects of CDNF can be protecting neurons from the toxic effects of α-synuclein. Here, we investigated the effects of CDNF on α-synuclein aggregation in vitro and in vivo. We found that CDNF directly interacts with α-synuclein with a KD = 23 ± 6 nM and reduces its auto-association. Using nuclear magnetic resonance (NMR) spectroscopy, we identified interaction sites on the CDNF protein. Remarkably, CDNF reduces the neuronal internalization of α-synuclein fibrils and induces the formation of insoluble phosphorylated α-synuclein inclusions. Intra-striatal CDNF administration alleviates motor deficits in rodents challenged with α-synuclein fibrils, though it did not reduce the number of phosphorylated α-synuclein inclusions in the substantia nigra. CDNF's beneficial effects on rodent behavior appear not to be related to the number of inclusions formed in the current context, and further study of its effects on the aggregation mechanism in vivo are needed. Nonetheless, the interaction of CDNF with α-synuclein, modifying its aggregation, spreading, and associated behavioral alterations, provides novel insights into the potential of CDNF as a therapeutic strategy in PD and other synucleinopathies.
Keywords: CDNF; MANF; Parkinson’s disease; cerebral dopamine neurotrophic factor; mesencephalic astrocyte-derived neurotrophic factor; pre-formed α-synuclein fibrils; protein aggregation; synucleinopathy; α-synuclein.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.S. is the founder and shareholder of Herantis Pharma Plc. H.J.H. is an employee and a shareholder of Herantis Pharma Plc. A.D. is an employee of Orion Corporation.
Figures

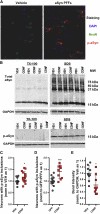
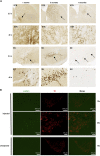

Comment in
-
CDNF: An innovative actor in disease-modifying approaches for Parkinson's disease.Mol Ther. 2021 Sep 1;29(9):2634-2636. doi: 10.1016/j.ymthe.2021.08.015. Mol Ther. 2021. PMID: 34473959 Free PMC article. No abstract available.
References
-
- Fearnley J.M., Lees A.J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. - PubMed
-
- Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. - PubMed
-
- Gibb W.R.G., Lees A.J. The significance of the Lewy body in the diagnosis of idiopathic Parkinson’s disease. Neuropathol. Appl. Neurobiol. 1989;15:27–44. - PubMed
-
- Spillantini M.G., Schmidt M.L., Lee V.M.-Y., Trojanowski J.Q., Jakes R., Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

