The outcome of a national MS-Covid-19 study: What the Turkish MS cohort reveals?
- PMID: 33940495
- PMCID: PMC8053402
- DOI: 10.1016/j.msard.2021.102968
The outcome of a national MS-Covid-19 study: What the Turkish MS cohort reveals?
Abstract
Background: The pandemic of the new type of corona virus infection 2019 [Covid-19] also affect people with Multiple Sclerosis (pwMS). Currently, the accumulating information on the effects of the infection regarding the demographic and clinical characteristics of the disease, as well as outcomes within different DMTs¸ enable us to have better practices on the management of the Covid-19 infection in pwMS.
Objective: To investigate the incidence of coronavirus disease 2019 (Covid-19) and to reveal the relationship between the demographic-clinical and therapeutic features and the outcome of Covid-19 infection in a multi-center national cohort of pwMS.
Methods: The Turkish Neurological Society-MS Study Group in association with the Italian MuSC-19 Study Group initiated this study. A web-based electronic Case Report Form (eCRF) of Study-MuSC-19 were used to collect the data. The demographic data and MS histories of the patients were obtained from the file tracking forms of the relevant clinics.
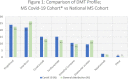
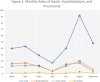
Results: 309 MS patients with confirmed Covid-19 infection were included in this study. Two hundred nineteen (219) were females (70.9%). The mean age was 36.9, ranging from 18 to 66, 194 of them (62.8%) were under 40. The clinical phenotype was relapsing-remitting in 277 (89.6%) and progressive in 32 (10.4%). Disease duration ranged from 0.2 years to 31.4 years. The median EDSS was 1.5, ranging from 0 to 8.5. The EDSS score was<= 1 in 134 (43%) of the patients. 91.6% of the patients were on a DMT, Fingolimod was the most frequently used drug (22.0%), followed by Interferon (20.1%). The comorbidity rate is 11.7%. We were not able to detect any significant association of DMTs with Covid-19 severity.
Conclusion: The Turkish MS-Covid-19 cohort had confirmed that pwMS are not at risk of having a more severe COVID-19 outcome irrespective of the DMT that they are treated. In addition, due to being a younger population with less comorbidities most had a mild disease further highlight that the only associated risk factors for having a moderate to severe COVID-19 course are similar with the general population such as having comorbid conditions and being older.
Keywords: Coronavirus; Disease modifying treatment; Disease severity; Multiple Sclerosis.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
S. Sen has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma.
Rana Karabudak has received honoraria for giving educational lectures, consultancy fees for participating advisory boards, and travel grants for attending scientific congresses or symposia from Roche, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey, Abdi İbrahim İlac, Deva and ARIS.
H. Efendi has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi İbrahim İlac.
A Siva has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi İbrahim İlac.
The rest of authors declare no conflict of interest with the study project.
Figures
References
-
- Akiyama S, Hamdeh S, Micic D, et al. Prevalence and clinical outcomes of Covid-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann. Rheum. Dis. 2020 Oct 13;annrheumdis-2020-218946. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

