Sphingolipid lysosomal storage diseases: from bench to bedside
- PMID: 33941173
- PMCID: PMC8094529
- DOI: 10.1186/s12944-021-01466-0
Sphingolipid lysosomal storage diseases: from bench to bedside
Abstract
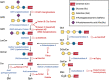
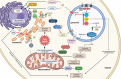
Johann Ludwig Wilhelm Thudicum described sphingolipids (SLs) in the late nineteenth century, but it was only in the past fifty years that SL research surged in importance and applicability. Currently, sphingolipids and their metabolism are hotly debated topics in various biochemical fields. Similar to other macromolecular reactions, SL metabolism has important implications in health and disease in most cells. A plethora of SL-related genetic ailments has been described. Defects in SL catabolism can cause the accumulation of SLs, leading to many types of lysosomal storage diseases (LSDs) collectively called sphingolipidoses. These diseases mainly impact the neuronal and immune systems, but other systems can be affected as well. This review aims to present a comprehensive, up-to-date picture of the rapidly growing field of sphingolipid LSDs, their etiology, pathology, and potential therapeutic strategies. We first describe LSDs biochemically and briefly discuss their catabolism, followed by general aspects of the major diseases such as Gaucher, Krabbe, Fabry, and Farber among others. We conclude with an overview of the available and potential future therapies for many of the diseases. We strive to present the most important and recent findings from basic research and clinical applications, and to provide a valuable source for understanding these disorders.
Keywords: Fabry; Gaucher; Krabbe; gangliosidosis; inborn errors of metabolism; lysosomal storage diseases; neurological diseases; sphingolipidoses; sphingolipids.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Kolter T, Sandhoff K. Lysosomal degradation of membrane lipids. FEBS Lett. 2010;584:1700–1712. - PubMed
-
- Albeituni S, Stiban J. Roles of Ceramides and Other Sphingolipids in Immune Cell Function and Inflammation. Adv Exp Med Biol. 2019;1161:169–191. - PubMed
-
- Futerman A. Biochemistry of Lipids, Lipoproteins and Membranes 6th Edition. 2016. Sphingolipids; pp. 297–326.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

