Pyroptosis: a new paradigm of cell death for fighting against cancer
- PMID: 33941231
- PMCID: PMC8091792
- DOI: 10.1186/s13046-021-01959-x
Pyroptosis: a new paradigm of cell death for fighting against cancer
Erratum in
-
Correction to: Pyroptosis: a new paradigm of cell death for fighting against cancer.J Exp Clin Cancer Res. 2021 Jul 1;40(1):219. doi: 10.1186/s13046-021-02020-7. J Exp Clin Cancer Res. 2021. PMID: 34210329 Free PMC article. No abstract available.
-
Correction to: Pyroptosis: a new paradigm of cell death for fighting against cancer.J Exp Clin Cancer Res. 2021 Sep 22;40(1):296. doi: 10.1186/s13046-021-02101-7. J Exp Clin Cancer Res. 2021. PMID: 34551788 Free PMC article. No abstract available.
Abstract
Background: Unraveling the mystery of cell death is one of the most fundamental progresses of life sciences during the past decades. Regulated cell death (RCD) or programmed cell death (PCD) is not only essential in embryonic development, but also plays an important role in the occurrence and progression of diseases, especially cancers. Escaping of cell death is one of hallmarks of cancer.
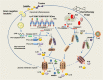
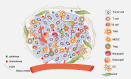
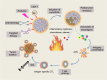
Main body: Pyroptosis is an inflammatory cell death usually caused by microbial infection, accompanied by activation of inflammasomes and maturation of pro-inflammatory cytokines interleukin-1β (IL-1β) and interleukin-18 (IL-18). Gasdermin family proteins are the executors of pyroptosis. Cytotoxic N-terminal of gasdermins generated from caspases or granzymes proteases mediated cleavage of gasdermin proteins oligomerizes and forms pore across cell membrane, leading to release of IL-1β, IL-18. Pyroptosis exerts tumor suppression function and evokes anti-tumor immune responses. Therapeutic regimens, including chemotherapy, radiotherapy, targeted therapy and immune therapy, induce pyroptosis in cancer, which potentiate local and systemic anti-tumor immunity. On the other hand, pyroptosis of normal cells attributes to side effects of anti-cancer therapies.
Conclusion: In this review, we focus on the regulatory mechanisms of pyroptosis and the tumor suppressive function of pyroptosis. We discuss the attribution of pyroptosis in reprogramming tumor microenvironments and restoration of anti-tumor immunity and its potential application in cancer immune therapy.
Keywords: Adaptive immunity; Ferroptosis; Gasdermin; Immune checkpoint; Immunogenic cell death; Necroptosis; Tumor microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
-
- Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15(2):135–147. - PubMed
-
- Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, Bracci L, Breckpot K, Brough D, Buqué A, Castro MG, Cirone M, Colombo MI, Cremer I, Demaria S, Dini L, Eliopoulos AG, Faggioni A, Formenti SC, Fučíková J, Gabriele L, Gaipl US, Galon J, Garg A, Ghiringhelli F, Giese NA, Guo ZS, Hemminki A, Herrmann M, Hodge JW, Holdenrieder S, Honeychurch J, Hu HM, Huang X, Illidge TM, Kono K, Korbelik M, Krysko DV, Loi S, Lowenstein PR, Lugli E, Ma Y, Madeo F, Manfredi AA, Martins I, Mavilio D, Menger L, Merendino N, Michaud M, Mignot G, Mossman KL, Multhoff G, Oehler R, Palombo F, Panaretakis T, Pol J, Proietti E, Ricci JE, Riganti C, Rovere-Querini P, Rubartelli A, Sistigu A, Smyth MJ, Sonnemann J, Spisek R, Stagg J, Sukkurwala AQ, Tartour E, Thorburn A, Thorne SH, Vandenabeele P, Velotti F, Workenhe ST, Yang H, Zong WX, Zitvogel L, Kroemer G, Galluzzi L. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3(9):e955691. - PMC - PubMed
-
- Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17(4):262–275. - PubMed
-
- Legrand AJ, Konstantinou M, Goode EF, Meier P. The diversification of cell death and immunity: memento Mori. Mol Cell. 2019;76(2):232–242. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

