COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters
- PMID: 33941622
- PMCID: PMC8158965
- DOI: 10.1126/scitranslmed.abf8396
COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters
Abstract
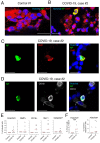
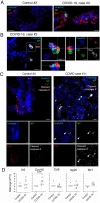
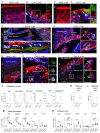
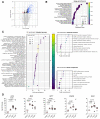
Whereas recent investigations have revealed viral, inflammatory, and vascular factors involved in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) lung pathogenesis, the pathophysiology of neurological disorders in coronavirus disease 2019 (COVID-19) remains poorly understood. Olfactory and taste dysfunction are common in COVID-19, especially in mildly symptomatic patients. Here, we conducted a virologic, molecular, and cellular study of the olfactory neuroepithelium of seven patients with COVID-19 presenting with acute loss of smell. We report evidence that the olfactory neuroepithelium is a major site of SARS-CoV2 infection with multiple cell types, including olfactory sensory neurons, support cells, and immune cells, becoming infected. SARS-CoV-2 replication in the olfactory neuroepithelium was associated with local inflammation. Furthermore, we showed that SARS-CoV-2 induced acute anosmia and ageusia in golden Syrian hamsters, lasting as long as the virus remained in the olfactory epithelium and the olfactory bulb. Last, olfactory mucosa sampling from patients showing long-term persistence of COVID-19-associated anosmia revealed the presence of virus transcripts and of SARS-CoV-2-infected cells, together with protracted inflammation. SARS-CoV-2 persistence and associated inflammation in the olfactory neuroepithelium may account for prolonged or relapsing symptoms of COVID-19, such as loss of smell, which should be considered for optimal medical management of this disease.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures



Similar articles
-
Regeneration Profiles of Olfactory Epithelium after SARS-CoV-2 Infection in Golden Syrian Hamsters.ACS Chem Neurosci. 2021 Feb 17;12(4):589-595. doi: 10.1021/acschemneuro.0c00649. Epub 2021 Feb 1. ACS Chem Neurosci. 2021. PMID: 33522795 Free PMC article.
-
Recovery of anosmia in hamsters infected with SARS-CoV-2 is correlated with repair of the olfactory epithelium.Sci Rep. 2022 Jan 12;12(1):628. doi: 10.1038/s41598-021-04622-9. Sci Rep. 2022. PMID: 35022504 Free PMC article.
-
Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters.Brain Behav Immun. 2020 Oct;89:579-586. doi: 10.1016/j.bbi.2020.06.032. Epub 2020 Jul 3. Brain Behav Immun. 2020. PMID: 32629042 Free PMC article.
-
The Molecular Basis of Olfactory Dysfunction in COVID-19 and Long COVID.Lifestyle Genom. 2024;17(1):42-56. doi: 10.1159/000539292. Epub 2024 May 15. Lifestyle Genom. 2024. PMID: 38749402 Review.
-
COVID-19 Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2?Viruses. 2021 Nov 4;13(11):2225. doi: 10.3390/v13112225. Viruses. 2021. PMID: 34835030 Free PMC article. Review.
Cited by
-
Cognitive impairments in patients with subacute coronavirus disease: Initial experiences in a post-coronavirus disease clinic.Front Aging Neurosci. 2022 Nov 9;14:994331. doi: 10.3389/fnagi.2022.994331. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36437985 Free PMC article.
-
SARS-CoV-2 cellular tropism and direct multiorgan failure in COVID-19 patients: Bioinformatic predictions, experimental observations, and open questions.Cell Biol Int. 2023 Feb;47(2):308-326. doi: 10.1002/cbin.11928. Epub 2022 Oct 13. Cell Biol Int. 2023. PMID: 36229927 Free PMC article. Review.
-
In vitro and in vivo differences in neurovirulence between D614G, Delta And Omicron BA.1 SARS-CoV-2 variants.Acta Neuropathol Commun. 2022 Sep 5;10(1):124. doi: 10.1186/s40478-022-01426-4. Acta Neuropathol Commun. 2022. PMID: 36058935 Free PMC article.
-
Possible Combinatorial Utilization of Phytochemicals and Extracellular Vesicles for Wound Healing and Regeneration.Int J Mol Sci. 2024 Sep 26;25(19):10353. doi: 10.3390/ijms251910353. Int J Mol Sci. 2024. PMID: 39408681 Free PMC article. Review.
-
SARS-CoV and SARS-CoV-2 display limited neuronal infection and lack the ability to transmit within synaptically connected axons in stem cell-derived human neurons.J Neurovirol. 2024 Feb;30(1):39-51. doi: 10.1007/s13365-023-01187-3. Epub 2024 Jan 3. J Neurovirol. 2024. PMID: 38172412 Free PMC article.
References
-
- WHO, WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ (2020).
-
- Qiu C., Cui C., Hautefort C., Haehner A., Zhao J., Yao Q., Zeng H., Nisenbaum E. J., Liu L., Zhao Y., Zhang D., Levine C. G., Cejas I., Dai Q., Zeng M., Herman P., Jourdaine C., de With K., Draf J., Chen B., Jayaweera D. T., Denneny J. C. 3rd, Casiano R., Yu H., Eshraghi A. A., Hummel T., Liu X., Shu Y., Lu H., Olfactory and Gustatory Dysfunction as an Early Identifier of COVID-19 in Adults and Children: An International Multicenter Study. Otolaryngol. Head Neck Surg. 163, 714–721 (2020). 10.1177/0194599820934376 - DOI - PMC - PubMed
-
- Cantuti-Castelvetri L., Ojha R., Pedro L. D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Österlund P., Joensuu M., Meunier F. A., Butcher S. J., Winkler M. S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M., Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

