Manipulating placebo analgesia and nocebo hyperalgesia by changing brain excitability
- PMID: 33941677
- PMCID: PMC8126770
- DOI: 10.1073/pnas.2101273118
Manipulating placebo analgesia and nocebo hyperalgesia by changing brain excitability
Abstract
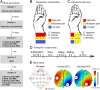
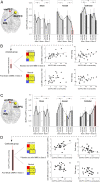
Harnessing placebo and nocebo effects has significant implications for research and medical practice. Placebo analgesia and nocebo hyperalgesia, the most well-studied placebo and nocebo effects, are thought to initiate from the dorsal lateral prefrontal cortex (DLPFC) and then trigger the brain's descending pain modulatory system and other pain regulation pathways. Combining repeated transcranial direct current stimulation (tDCS), an expectancy manipulation model, and functional MRI, we investigated the modulatory effects of anodal and cathodal tDCS at the right DLPFC on placebo analgesia and nocebo hyperalgesia using a randomized, double-blind and sham-controlled design. We found that compared with sham tDCS, active tDCS could 1) boost placebo and blunt nocebo effects and 2) modulate brain activity and connectivity associated with placebo analgesia and nocebo hyperalgesia. These results provide a basis for mechanistic manipulation of placebo and nocebo effects and may lead to improved clinical outcomes in medical practice.
Keywords: dorsolateral prefrontal cortex; expectancy manipulation; mechanistic manipulation; placebo and nocebo effects; transcranial direct current stimulation.
Conflict of interest statement
Competing interest statement: J.K. holds equity in a startup company (MNT) and a pending patent to develop new peripheral neuromodulation tools, but declares no conflict of interest. All other authors declare no conflict of interest.
Figures

References
-
- Colloca L., Barsky A. J., Placebo and nocebo effects. N. Engl. J. Med. 382, 554–561 (2020). - PubMed
-
- Hoffman G. A., Harrington A., Fields H. L., Pain and the placebo: What we have learned. Perspect. Biol. Med. 48, 248–265 (2005). - PubMed
-
- Kong J., Kaptchuk T. J., Polich G., Kirsch I., Gollub R. L., Placebo analgesia: Findings from brain imaging studies and emerging hypotheses. Rev. Neurosci. 18, 173–190 (2007). - PubMed
-
- Enck P., Benedetti F., Schedlowski M., New insights into the placebo and nocebo responses. Neuron 59, 195–206 (2008). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

