Reversed-engineered human alveolar lung-on-a-chip model
- PMID: 33941687
- PMCID: PMC8126776
- DOI: 10.1073/pnas.2016146118
Reversed-engineered human alveolar lung-on-a-chip model
Abstract
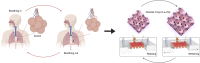
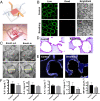
Here, we present a physiologically relevant model of the human pulmonary alveoli. This alveolar lung-on-a-chip platform is composed of a three-dimensional porous hydrogel made of gelatin methacryloyl with an inverse opal structure, bonded to a compartmentalized polydimethylsiloxane chip. The inverse opal hydrogel structure features well-defined, interconnected pores with high similarity to human alveolar sacs. By populating the sacs with primary human alveolar epithelial cells, functional epithelial monolayers are readily formed. Cyclic strain is integrated into the device to allow biomimetic breathing events of the alveolar lung, which, in addition, makes it possible to investigate pathological effects such as those incurred by cigarette smoking and severe acute respiratory syndrome coronavirus 2 pseudoviral infection. Our study demonstrates a unique method for reconstitution of the functional human pulmonary alveoli in vitro, which is anticipated to pave the way for investigating relevant physiological and pathological events in the human distal lung.
Keywords: alveoli; distal lung; inverse opal; lung-on-a-chip; three-dimensional.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Papi A., Brightling C., Pedersen S. E., Reddel H. K., Asthma. Lancet 391, 783–800 (2018). - PubMed
-
- López-Campos J. L., Tan W., Soriano J. B., Global burden of COPD. Respirology 21, 14–23 (2016). - PubMed
-
- Mandell L. A., Niederman M. S., Aspiration pneumonia. N. Engl. J. Med. 380, 651–663 (2019). - PubMed
-
- Reid M. J. A., et al. ., Building a tuberculosis-free world: The Lancet Commission on tuberculosis. Lancet 393, 1331–1384 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

