Membrane fusion and drug delivery with carbon nanotube porins
- PMID: 33941689
- PMCID: PMC8126853
- DOI: 10.1073/pnas.2016974118
Membrane fusion and drug delivery with carbon nanotube porins
Abstract
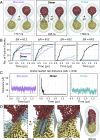
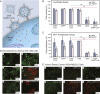
Drug delivery mitigates toxic side effects and poor pharmacokinetics of life-saving therapeutics and enhances treatment efficacy. However, direct cytoplasmic delivery of drugs and vaccines into cells has remained out of reach. We find that liposomes studded with 0.8-nm-wide carbon nanotube porins (CNTPs) function as efficient vehicles for direct cytoplasmic drug delivery by facilitating fusion of lipid membranes and complete mixing of the membrane material and vesicle interior content. Fusion kinetics data and coarse-grained molecular dynamics simulations reveal an unusual mechanism where CNTP dimers tether the vesicles, pull the membranes into proximity, and then fuse their outer and inner leaflets. Liposomes containing CNTPs in their membranes and loaded with an anticancer drug, doxorubicin, were effective in delivering the drug to cancer cells, killing up to 90% of them. Our results open an avenue for designing efficient drug delivery carriers compatible with a wide range of therapeutics.
Keywords: carbon nanotube porins; drug delivery; liposomes; membrane fusion.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: Some of the results reported in the paper have been disclosed as part of a provisional patent filing.
Figures

References
-
- Katzung B. G.. Basic & Clinical Pharmacology (McGraw-Hill, New York, ed. 14, 2018).
-
- Chabner B. A., Roberts T. G., Chemotherapy and the war on cancer. Nat. Rev. Canc. 5 (1), 65–72 (2005). - PubMed
-
- Timko B. P., et al. , Advances in drug delivery. Annu. Rev. Mater. Res. 41, 1–20 (2011).
-
- Rosen Y., Gurman P., Elman N., Drug Delivery: An Integrated Clinical and Engineering Approach (CRC Press, 2017).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

