Clinical use of the SelectMDx urinary-biomarker test with or without mpMRI in prostate cancer diagnosis: a prospective, multicenter study in biopsy-naïve men
- PMID: 33941866
- PMCID: PMC8616754
- DOI: 10.1038/s41391-021-00367-8
Clinical use of the SelectMDx urinary-biomarker test with or without mpMRI in prostate cancer diagnosis: a prospective, multicenter study in biopsy-naïve men
Abstract
Background: Risk stratification in men with suspicion of prostate cancer (PCa) requires reliable diagnostic tests, not only to identify high-grade PCa, also to minimize the overdetection of low-grade PCa, and reduction of "unnecessary" prostate MRIs and biopsies. This study aimed to evaluate the SelectMDx test to detect high-grade PCa in biopsy-naïve men. Subsequently, to assess combinations of SelectMDx test and multi-parametric (mp) MRI and its potential impact on patient selection for prostate biopsy.
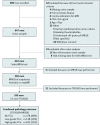
Methods: This prospective multicenter diagnostic study included 599 biopsy-naïve patients with prostate-specific antigen level ≥3 ng/ml. All patients underwent a SelectMDx test and mpMRI before systematic transrectal ultrasound-guided biopsy (TRUSGB). Patients with a suspicious mpMRI also had an in-bore MR-guided biopsy (MRGB). Histopathologic outcome of TRUSGB and MRGB was used as reference standard. High-grade PCa was defined as ISUP Grade Group (GG) ≥ 2. The primary outcome was the detection rates of low- and high-grade PCa and number of biopsies avoided in four strategies, i.e., (1) SelectMDx test-only, (2) mpMRI-only, (3) SelectMDx test followed by mpMRI when SelectMDx test was positive (conditional strategy), and (4) SelectMDx test and mpMRI in all (joint strategy). A positive SelectMDx test outcome was a risk score of ≥-2.8. Decision curve analysis (DCA) was performed to assess clinical utility.
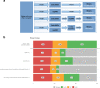
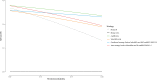
Results: Prevalence of high-grade PCa was 31% (183/599). Thirty-eight percent (227/599) of patients had negative SelectMDx test in whom biopsy could be avoided. Low-grade PCa was not detected in 35% (48/138) with missing 10% (18/183) high-grade PCa. Yet, mpMRI-only could avoid 49% of biopsies, not detecting 4.9% (9/183) of high-grade PCa. The conditional strategy reduces the number of mpMRIs by 38% (227/599), avoiding biopsy in 60% (357/599) and missing 13% (24/183) high-grade PCa. Low-grade PCa was not detected in 58% (80/138). DCA showed the highest net benefit for the mpMRI-only strategy, followed by the conditional strategy at-risk thresholds >10%.
Conclusions: SelectMDx test as a risk stratification tool for biopsy-naïve men avoids unnecessary biopsies in 38%, minimizes low-grade PCa detection, and misses only 10% high-grade PCa. Yet, using mpMRI in all patients had the highest net benefit, avoiding biopsy in 49% and missing 4.9% of high-risk PCa. However, if mpMRI availability is limited or expensive, using mpMRI-only in SelectMDx test positive patients is a good alternative strategy.
© 2021. The Author(s).
Conflict of interest statement
IMO and JOB certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g., employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: JAS, WVC, HJ, and EDC are employees of or consultants for MDxHealth and may have stock or stock options, IMO is a member of the editorial board of the website prostateMDx.org, owned by MDxHealth. The other authors declare no competing interests.
Figures

References
-
- Roobol MJ, Verbeek JFM, van der Kwast T, Kummerlin IP, Kweldam CF, van Leenders G. Improving the Rotterdam European Randomized Study of screening for prostate cancer risk calculator for initial prostate biopsy by incorporating the 2014 International Society of Urological Pathology Gleason grading and cribriform growth. Eur Urol. 2017;72:45–51. doi: 10.1016/j.eururo.2017.01.033. - DOI - PubMed
-
- Rouviere O, Puech P, Renard-Penna R, Claudon M, Roy C, Mege-Lechevallier F, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20:100–9. doi: 10.1016/S1470-2045(18)30569-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

