Long-term outcomes of intravitreal therapy for symptomatic diabetic macular oedema in a real-world setting in Switzerland
- PMID: 33942162
- PMCID: PMC8589751
- DOI: 10.1007/s00417-021-05187-z
Long-term outcomes of intravitreal therapy for symptomatic diabetic macular oedema in a real-world setting in Switzerland
Abstract
Objective: To assess the long-term visual outcomes in eyes with symptomatic diabetic macular oedema (DME) under intravitreal treatment (IVT) in a clinical routine setting.
Methods: Patients with newly diagnosed DME were included in this retrospective study if they had received at least three IVTs and a follow-up period ≥ 2 years. Due to altered treatment patterns since the approval of ranibizumab for DME in 2012, patients were subdivided according to their first IVT before 2013 (group 1) or thereafter (group 2). The primary outcome measure was the evolution of best-corrected visual acuity (BCVA) over time.
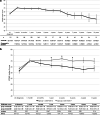
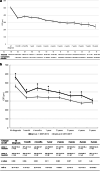
Results: Of 217 eyes (191 patients) with DME, 151 eyes (117 patients) fulfilled the inclusion criteria (63 eyes in the first period, 88 in the second period). Mean follow-up time was 7.9 ± 3.1 (group 1) and 4.1 ± 1.4 years (group 2; p < 0.001). Visual gains were similar in the first year (group 1: + 5.3 ± 15.5, group 2: + 7.3 ± 12.2 Early Treatment Diabetic Retinopathy Study (ETDRS) letters; p = 0.44), but not thereafter (after 2 years in group 1: + 4.4 ± 15.0, group 2: + 8.3 ± 13.0 ETDRS letters; p = 0.038). During the first year, group 1 patients received less clinical examinations (group 1: 6.6 ± 3.3, group 2: 7.5 ± 2.1; p = 0.007) and less injections (group 1: 3.6 ± 2.7, group 2: 6.1 ± 2.7; p < 0.001).
Conclusion: A greater visual gain, in response to more intensive treatment during the first year, was maintained for at least 5 years in group 2 subjects. Our data confirm that in a real-world setting, early intensive treatment results in satisfying long-term visual outcomes.
Keywords: Aflibercept; Anti-VEGF; Consecutive case series; Diabetic macular oedema (DME); Long-term treatment; Ranibizumab; Real-world studies.
© 2021. The Author(s).
Conflict of interest statement
JGG acts as advisor for several pharmaceutical companies and contributes to several clinical studies. The underlying manuscript is independent of these activities. The author did not receive direct or indirect support for this study and does not have conflicting interests with the data that are presented herein. None of the other authors reports any potential conflict of interest.
Figures
Similar articles
-
Prospective study of aflibercept for the treatment of persistent macular oedema secondary to retinal vein occlusions in eyes not responsive to long-term treatment with bevacizumab or ranibizumab.Clin Exp Ophthalmol. 2020 Jan;48(1):53-60. doi: 10.1111/ceo.13636. Epub 2019 Oct 10. Clin Exp Ophthalmol. 2020. PMID: 31498950 Clinical Trial.
-
Real-world management of treatment-naïve diabetic macular oedema in Japan: two-year visual outcomes with and without anti-VEGF therapy in the STREAT-DME study.Br J Ophthalmol. 2020 Sep;104(9):1209-1215. doi: 10.1136/bjophthalmol-2019-315199. Epub 2019 Nov 29. Br J Ophthalmol. 2020. PMID: 31784500 Free PMC article.
-
Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial.JAMA Ophthalmol. 2018 Mar 1;136(3):257-269. doi: 10.1001/jamaophthalmol.2017.6565. JAMA Ophthalmol. 2018. PMID: 29392288 Free PMC article. Clinical Trial.
-
Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net Protocol T.Curr Opin Ophthalmol. 2017 Nov;28(6):636-643. doi: 10.1097/ICU.0000000000000424. Curr Opin Ophthalmol. 2017. PMID: 28837425 Review.
-
Recent clinically relevant highlights from the Diabetic Retinopathy Clinical Research Network.Curr Opin Ophthalmol. 2018 May;29(3):199-205. doi: 10.1097/ICU.0000000000000472. Curr Opin Ophthalmol. 2018. PMID: 29528861 Review.
Cited by
-
Functional Outcomes of Brolucizumab-Induced Intraocular Inflammation Involving the Posterior Segment-A Meta-Analysis and Systematic Review.J Clin Med. 2023 Jul 14;12(14):4671. doi: 10.3390/jcm12144671. J Clin Med. 2023. PMID: 37510788 Free PMC article. Review.
-
Visual and anatomical failure of anti-VEGF therapy for retinal vascular diseases: a survival analysis of real-world data.Eye (Lond). 2025 Apr;39(5):977-985. doi: 10.1038/s41433-024-03529-9. Epub 2024 Dec 10. Eye (Lond). 2025. PMID: 39658713 Free PMC article.
-
Brolucizumab in Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: Ophthalmology and Diabetology Treatment Aspects.Ophthalmol Ther. 2023 Apr;12(2):639-655. doi: 10.1007/s40123-023-00647-7. Epub 2023 Jan 12. Ophthalmol Ther. 2023. PMID: 36633780 Free PMC article.
-
Visual outcomes of observation, macular laser and anti-VEGF in diabetic macular edema in type 1 diabetes: a real-world study.BMC Ophthalmol. 2022 Jun 9;22(1):258. doi: 10.1186/s12886-022-02482-z. BMC Ophthalmol. 2022. PMID: 35681133 Free PMC article.
-
Anti-VEGF Treatment of Diabetic Macular Edema in Denmark: Incidence, Burden of Therapy, and Forecasting Analyses.J Pers Med. 2023 Mar 18;13(3):546. doi: 10.3390/jpm13030546. J Pers Med. 2023. PMID: 36983726 Free PMC article.
References
-
- Holekamp NM (2016) Overview of diabetic macular edema. Am J Manag Care. 22(10 Suppl):s284-s291. - PubMed
-
- Leasher JL, Bourne RR, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease Study Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39(9):1643–1649. doi: 10.2337/dc15-2171. - DOI - PubMed
-
- Jan S, Nazim M, Karim S, Hussain Z. Intravitreal bevacizumab: Indications and complications. J Ayub Med Coll Abbottabad. 2016;28(2):364–368. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

