Exploring Risks of Human Challenge Trials For COVID-19
- PMID: 33942351
- PMCID: PMC8207107
- DOI: 10.1111/risa.13726
Exploring Risks of Human Challenge Trials For COVID-19
Abstract
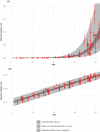
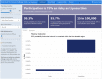
Human challenge trials (HCTs) are a potential method to accelerate development of vaccines and therapeutics. However, HCTs for COVID-19 pose ethical and practical challenges, in part due to the unclear and developing risks. In this article , we introduce an interactive model for exploring some risks of a severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) dosing study, a prerequisite for any COVID-19 challenge trials. The risk estimates we use are based on a Bayesian evidence synthesis model which can incorporate new data on infection fatality risks (IFRs) to patients, and infer rates of hospitalization. The model estimates individual risk, which we then extrapolate to overall mortality and hospitalization risk in a dosing study. We provide a web tool to explore risk under different study designs. Based on the Bayesian model, IFR for someone between 20 and 30 years of age is 15.1 in 100,000, with a 95% uncertainty interval from 11.8 to 19.2, while risk of hospitalization is 130 per 100,000 (100-160). However, risk will be reduced in an HCT via screening for comorbidities, selecting lower-risk population, and providing treatment. Accounting for this with stronger assumptions, we project the fatality risk to be as low as 2.5 per 100,000 (1.6-3.9) and the hospitalization risk to be 22.0 per 100,000 (14.0-33.7). We therefore find a 50-person dosing trial has a 99.74% (99.8-99.9%) chance of no fatalities, and a 98.9% (98.3-99.3%) probability of no cases requiring hospitalization.
Keywords: Bayesian meta-analysis; COVID-19; human challenge trial; informed consent; interactive models; risk communication.
© 2021 The Authors. Risk Analysis published by Wiley Periodicals LLC on behalf of Society for Risk Analysis.
Figures
References
-
- 1Day Sooner . (2020). 1Day Sooner web site. Retrieved from https://1daysooner.org/
-
- Agrawal, G. , Conway, M. , Heller, J. , Sabow, A. , & Tolub, G. (2020). On pins and needles: Will COVID‐19 vaccines save the world? (Tech. Rep.). Online: McKinsey & Company. https://www.mckinsey.com/~/media/mckinsey/industries/pharmaceuticals%20a....
-
- Ahmad, N. , Peterson, N. , & Torella, F. (2015). The micromort: A unit for comparing and communicating risk to patients. International Journal of Clinical Practice, 69(5), 515–517. - PubMed
-
- Betancourt, M. (2016). Logic, probability, and Bayesian inference: A conceptual introduction to the foundations of applied inference for scientists and engineers (Tech. Rep.). The Stan Development Team. Retrieved from https://github.com/betanalpha/stan_intro/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

