The detrimental effect of iron on OA chondrocytes: Importance of pro-inflammatory cytokines induced iron influx and oxidative stress
- PMID: 33942503
- PMCID: PMC8184674
- DOI: 10.1111/jcmm.16581
The detrimental effect of iron on OA chondrocytes: Importance of pro-inflammatory cytokines induced iron influx and oxidative stress
Abstract
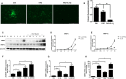
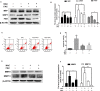
Iron overload is common in elderly people which is implicated in the disease progression of osteoarthritis (OA), however, how iron homeostasis is regulated during the onset and progression of OA and how it contributes to the pathological transition of articular chondrocytes remain unknown. In the present study, we developed an in vitro approach to investigate the roles of iron homeostasis and iron overload mediated oxidative stress in chondrocytes under an inflammatory environment. We found that pro-inflammatory cytokines could disrupt chondrocytes iron homeostasis via upregulating iron influx transporter TfR1 and downregulating iron efflux transporter FPN, thus leading to chondrocytes iron overload. Iron overload would promote the expression of chondrocytes catabolic markers, MMP3 and MMP13 expression. In addition, we found that oxidative stress and mitochondrial dysfunction played important roles in iron overload-induced cartilage degeneration, reducing iron concentration using iron chelator or antioxidant drugs could inhibit iron overload-induced OA-related catabolic markers and mitochondrial dysfunction. Our results suggest that pro-inflammatory cytokines could disrupt chondrocytes iron homeostasis and promote iron influx, iron overload-induced oxidative stress and mitochondrial dysfunction play important roles in iron overload-induced cartilage degeneration.
Keywords: iron overload; mitochondrial dysfunction; osteoarthritis; oxidative stress; pro-inflammatory cytokines.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



Similar articles
-
Iron Overload Is Associated With Accelerated Progression of Osteoarthritis: The Role of DMT1 Mediated Iron Homeostasis.Front Cell Dev Biol. 2021 Jan 5;8:594509. doi: 10.3389/fcell.2020.594509. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33469535 Free PMC article.
-
Calcium chelator BAPTA‑AM protects against iron overload‑induced chondrocyte mitochondrial dysfunction and cartilage degeneration.Int J Mol Med. 2021 Oct;48(4):196. doi: 10.3892/ijmm.2021.5029. Epub 2021 Sep 1. Int J Mol Med. 2021. PMID: 34468013 Free PMC article.
-
IL37 dampens the IL1β-induced catabolic status of human OA chondrocytes.Rheumatology (Oxford). 2017 Mar 1;56(3):351-361. doi: 10.1093/rheumatology/kew411. Rheumatology (Oxford). 2017. PMID: 27940589
-
Mitochondrial pathology in osteoarthritic chondrocytes.Curr Drug Targets. 2014;15(7):710-9. doi: 10.2174/1389450115666140417120305. Curr Drug Targets. 2014. PMID: 24745822 Review.
-
Molecular regulation of articular chondrocyte function and its significance in osteoarthritis.Histol Histopathol. 2011 Mar;26(3):377-94. doi: 10.14670/HH-26.377. Histol Histopathol. 2011. PMID: 21210351 Review.
Cited by
-
Erythropoietin inhibits ferroptosis and ameliorates neurological function after spinal cord injury.Neural Regen Res. 2023 Apr;18(4):881-888. doi: 10.4103/1673-5374.353496. Neural Regen Res. 2023. PMID: 36204858 Free PMC article.
-
The Role Played by Ferroptosis in Osteoarthritis: Evidence Based on Iron Dyshomeostasis and Lipid Peroxidation.Antioxidants (Basel). 2022 Aug 27;11(9):1668. doi: 10.3390/antiox11091668. Antioxidants (Basel). 2022. PMID: 36139742 Free PMC article. Review.
-
Lipid peroxidation in osteoarthritis: focusing on 4-hydroxynonenal, malondialdehyde, and ferroptosis.Cell Death Discov. 2023 Aug 29;9(1):320. doi: 10.1038/s41420-023-01613-9. Cell Death Discov. 2023. PMID: 37644030 Free PMC article. Review.
-
Exploring the Causal Effects of Mineral Metabolism Disorders on Telomere and Mitochondrial DNA: A Bidirectional Two-Sample Mendelian Randomization Analysis.Nutrients. 2024 May 8;16(10):1417. doi: 10.3390/nu16101417. Nutrients. 2024. PMID: 38794655 Free PMC article.
-
Crosstalk between ferroptosis and chondrocytes in osteoarthritis: a systematic review of in vivo and in vitro studies.Front Immunol. 2023 Jul 14;14:1202436. doi: 10.3389/fimmu.2023.1202436. eCollection 2023. Front Immunol. 2023. PMID: 37520558 Free PMC article.
References
-
- Glyn‐Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376‐387. - PubMed
-
- Nieuwenhuizen L, Schutgens RE, van Asbeck BS, et al. Identification and expression of iron regulators in human synovium: evidence for upregulation in haemophilic arthropathy compared to rheumatoid arthritis, osteoarthritis, and healthy controls. Haemophilia. 2013;19(4):e218‐e227. - PubMed
-
- Roosendaal G, Tekoppele JM, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Articular cartilage is more susceptible to blood induced damage at young than at old age. J Rheumatol. 2000;27(7):1740‐1744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

