In Vivo Molecular K-Edge Imaging of Atherosclerotic Plaque Using Photon-counting CT
- PMID: 33944628
- PMCID: PMC8217298
- DOI: 10.1148/radiol.2021203968
In Vivo Molecular K-Edge Imaging of Atherosclerotic Plaque Using Photon-counting CT
Abstract
Background Macrophage burden is a major factor in the risk of atherosclerotic plaque rupture, and its evaluation remains challenging with molecular noninvasive imaging approaches. Photon-counting CT (PCCT) with k-edge imaging aims to allow for the specific detection of macrophages using gold nanoparticles. Purpose To perform k-edge imaging in combination with gold nanoparticles to detect and quantify the macrophage burden within the atherosclerotic aortas of rabbits. Materials and Methods Atherosclerotic and control New Zealand white rabbits were imaged before and at several time points up to 2 days after intravenous injection of gold nanoparticles (3.5 mL/kg, 65 mg gold per milliliter). Aortic CT angiography was performed at the end of the follow-up using an intravenous injection of an iodinated contrast material. Gold k-edge and conventional CT images were reconstructed for qualitative and quantitative assessment of the macrophage burden. PCCT imaging results were compared with findings at histologic examination, quantitative histomorphometry, transmission electron microscopy, and quantitative inductively coupled plasma optical emission spectrometry. Pearson correlations between the macrophage area measured in immunostained sections and the concentration of gold and attenuation measured in the corresponding PCCT sections were calculated. Results Seven rabbits with atherosclerosis and four control rabbits without atherosclerosis were analyzed. In atherosclerotic rabbits, calcifications were observed along the aortic wall before injection. At 2 days after injection of gold nanoparticles, only gold k-edge images allowed for the distinction of plaque enhancement within calcifications and for lumen enhancement during angiography. A good correlation was observed between the gold concentration measured within the wall and the macrophage area in 35 plaques (five per rabbit) (r = 0.82; 95% CI: 0.67, 0.91; P < .001), which was higher than that observed on conventional CT images (r = 0.41; 95% CI: 0.09, 0.65; P = .01). Transmission electron microscopy and inductively coupled plasma optical emission spectrometry analyses confirmed the gold k-edge imaging findings. Conclusion Photon-counting CT with gold nanoparticles allowed for the noninvasive evaluation of both molecular and anatomic information in vivo in rabbits with atherosclerotic plaques. Published under a CC BY 4.0 license. Online supplemental material is available for this article. See also the editorial by Leiner in this issue.
Conflict of interest statement
Figures


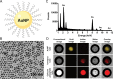

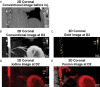
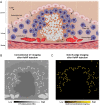

Comment in
-
A New Era in Atherosclerotic Plaque Characterization with Photon-counting CT.Radiology. 2021 Jul;300(1):108-109. doi: 10.1148/radiol.2021210313. Epub 2021 May 4. Radiology. 2021. PMID: 33949896 No abstract available.
References
-
- Mozaffarian D, Benjamin EJ, Go AS, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016;133(4):447–454. - PubMed
-
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108(14):1664–1672. - PubMed
-
- Libby P. Inflammation in atherosclerosis. Nature 2002;420(6917):868–874. - PubMed
-
- Prati F, Mallus MT, Broglia L, Albertucci M. Integrated non-invasive imaging techniques. EuroIntervention 2010;6(Suppl G):G161–G168. - PubMed
-
- Si-Mohamed S, Bar-Ness D, Sigovan M, et al. Review of an initial experience with an experimental spectral photon-counting computed tomography system. Nucl Instrum Methods Phys Res A 2017;873(27):35.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

