Effect of Therapeutic Drug Monitoring vs Standard Therapy During Infliximab Induction on Disease Remission in Patients With Chronic Immune-Mediated Inflammatory Diseases: A Randomized Clinical Trial
- PMID: 33944876
- PMCID: PMC8097498
- DOI: 10.1001/jama.2021.4172
Effect of Therapeutic Drug Monitoring vs Standard Therapy During Infliximab Induction on Disease Remission in Patients With Chronic Immune-Mediated Inflammatory Diseases: A Randomized Clinical Trial
Abstract
Importance: Proactive therapeutic drug monitoring (TDM), defined as individualized drug dosing based on scheduled monitoring of serum drug levels, has been proposed as an alternative to standard therapy to maximize efficacy and safety of infliximab and other biological drugs. However, whether proactive TDM improves clinical outcomes when implemented at the time of drug initiation, compared with standard therapy, remains unclear.
Objective: To assess whether TDM during initiation of infliximab therapy improves treatment efficacy compared with standard infliximab therapy without TDM.
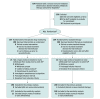
Design, setting, and participants: Randomized, parallel-group, open-label clinical trial of 411 adults with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, ulcerative colitis, Crohn disease, or psoriasis initiating infliximab therapy in 21 hospitals in Norway. Patients were recruited from March 1, 2017, to January 10, 2019. Final follow-up occurred on November 5, 2019.
Interventions: Patients were randomized 1:1 to receive proactive TDM with dose and interval adjustments based on scheduled monitoring of serum drug levels and antidrug antibodies (TDM group; n = 207) or standard infliximab therapy without drug and antibody level monitoring (standard therapy group; n = 204).
Main outcomes and measures: The primary end point was clinical remission at week 30.
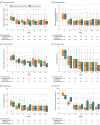
Results: Among 411 randomized patients (mean age, 44.7 [SD, 14.9] years; 209 women [51%]), 398 (198 in the TDM group and 200 in the standard therapy group) received their randomized intervention and were included in the full analysis set. Clinical remission at week 30 was achieved in 100 (50.5%) of 198 and 106 (53.0%) of 200 patients in the TDM and standard therapy groups, respectively (adjusted difference, 1.5%; 95% CI, -8.2% to 11.1%; P = .78). Adverse events were reported in 135 patients (68%) and 139 patients (70%) in the TDM and standard therapy groups, respectively.
Conclusions and relevance: Among patients with immune-mediated inflammatory diseases initiating treatment with infliximab, proactive therapeutic drug monitoring, compared with standard therapy, did not significantly improve clinical remission rates over 30 weeks. These findings do not support routine use of therapeutic drug monitoring during infliximab induction for improving disease remission rates.
Trial registration: ClinicalTrials.gov Identifier: NCT03074656.
Conflict of interest statement
Figures

Comment in
-
Treatment Strategies for Patients With Immune-Mediated Inflammatory Diseases.JAMA. 2021 May 4;325(17):1726-1728. doi: 10.1001/jama.2021.2740. JAMA. 2021. PMID: 33944889 No abstract available.
-
Therapeutic Drug Monitoring vs Standard Therapy During Infliximab Induction in Patients With Chronic Immune-Mediated Inflammatory Diseases.JAMA. 2021 Sep 21;326(11):1067. doi: 10.1001/jama.2021.11477. JAMA. 2021. PMID: 34546306 No abstract available.
-
Therapeutic Drug Monitoring vs Standard Therapy During Infliximab Induction in Patients With Chronic Immune-Mediated Inflammatory Diseases.JAMA. 2021 Sep 21;326(11):1069. doi: 10.1001/jama.2021.11474. JAMA. 2021. PMID: 34546307 No abstract available.
-
Therapeutic Drug Monitoring vs Standard Therapy During Infliximab Induction in Patients With Chronic Immune-Mediated Inflammatory Diseases.JAMA. 2021 Sep 21;326(11):1068-1069. doi: 10.1001/jama.2021.11471. JAMA. 2021. PMID: 34546308 No abstract available.
References
-
- Maini R, St Clair EW, Breedveld F, et al. ; ATTRACT Study Group . Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999;354(9194):1932-1939. doi:10.1016/S0140-6736(99)05246-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

