Docetaxel and cisplatin induction chemotherapy with or without fluorouracil in locoregionally advanced nasopharyngeal carcinoma: A retrospective propensity score matching analysis
- PMID: 33945215
- PMCID: PMC9291171
- DOI: 10.1111/ajco.13565
Docetaxel and cisplatin induction chemotherapy with or without fluorouracil in locoregionally advanced nasopharyngeal carcinoma: A retrospective propensity score matching analysis
Abstract
Purpose: To investigate whether the addition of fluorouracil to docetaxel and cisplatin induction chemotherapy (IC) can truly improve the prognosis of patients with locoregionally advanced nasopharyngeal carcinoma (NPC).
Methods: A total of 801 patients newly diagnosed with non-metastatic locoregionally advanced NPC were included as the subjects. In this study, propensity score matching (PSM) was used for analysis of overall survival (OS), distant metastasis-free survival (DMFS), progression-free survival (PFS) and locoregional relapse-free survival (LRRFS), and the chi-squared test or Fisher's exact test was used to investigate toxic reactions.
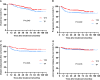
Results: Patients received treatment with docetaxel and cisplatin (TP) or docetaxel, cisplatin and fluorouracil (TPF). With a median follow-up time of 60 months (range: 5-124 months), the TPF group had better 5-year OS (84.7% vs 79.0%; P = 0.037), PFS (84.6% vs 76.8%; P = 0.008) and DMFS (89.5% vs 82.3%; P = 0.004) than the TP group. After PSM, 258 patients were matched in each cohort. The Kaplan-Meier analysis showed that the 5-year OS, PFS and DMFS were 85.5%, 84.2% and 89.2%, respectively, in the TPF group, higher than the 80.8%, 75.0% and 81.4%, respectively, in the TP group (P = 0.048, 0.009 and 0.006, respectively). Moreover, the multivariate analysis revealed that different IC regimens were independent prognostic factors for PFS and DMFS (P = 0.014 and 0.010, respectively).
Conclusion: This study found that compared with the TP regimen, TPF induction chemotherapy is associated with improved survival in patients with locoregionally advanced NPC. TPF can produce more mucosal and nausea/vomiting adverse reactions than TP.
Keywords: fluorouracil; induction chemotherapy; nasopharyngeal carcinoma; propensity score-matching.
© 2021 The Authors. Asia-Pacific Journal of Clinical Oncology published by John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Rong‐Hui Zheng and Tai‐Ze Yuan were guarantors of the entire study. Rong‐Hui Zheng, Tai‐Ze Yuan, Ze‐Jiang Zhan and Hao‐Yun Tao built up the study concepts and designed this study. Hao‐Yun Tao, Wen‐Ze Qiu and Kai Liao collected clinical data all needed. Wen‐Ze Qiu and Ya‐Wei Yuan performed the statistical analyses. Rong‐Hui Zheng, Tai‐Ze Yuan, Ze‐Jiang Zhan and Hao‐Yun Tao prepared and edited the manuscript. All authors read and approved the final manuscript.
No conflicts of interest exists.
Figures


Similar articles
-
Effect of induction chemotherapy with cisplatin, fluorouracil, with or without taxane on locoregionally advanced nasopharyngeal carcinoma: a retrospective, propensity score-matched analysis.Cancer Commun (Lond). 2018 May 10;38(1):21. doi: 10.1186/s40880-018-0283-2. Cancer Commun (Lond). 2018. PMID: 29764487 Free PMC article.
-
Long-term results of locoregionally advanced nasopharyngeal carcinoma treated with cisplatin and 5-fluorouracil induction chemotherapy with or without docetaxel in young and middle aged adults.J Cancer Res Clin Oncol. 2025 Mar 4;151(3):99. doi: 10.1007/s00432-025-06145-6. J Cancer Res Clin Oncol. 2025. PMID: 40035865 Free PMC article.
-
Clinical value of docetaxel plus cisplatin (TP) induction chemotherapy followed by TP concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma.J Cancer. 2021 Jan 1;12(1):18-27. doi: 10.7150/jca.49944. eCollection 2021. J Cancer. 2021. PMID: 33391399 Free PMC article.
-
Long-term outcomes of induction chemotherapy followed by concurrent chemoradiotherapy and adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: a retrospective study.Front Oncol. 2024 Nov 27;14:1475176. doi: 10.3389/fonc.2024.1475176. eCollection 2024. Front Oncol. 2024. PMID: 39664180 Free PMC article. Review.
-
The best choice of induction chemotherapy for patients with locally advanced nasopharyngeal carcinoma: Bayesian network meta-analysis.Head Neck. 2022 Feb;44(2):518-529. doi: 10.1002/hed.26932. Epub 2021 Dec 4. Head Neck. 2022. PMID: 34862812 Review.
Cited by
-
Induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: A systematic review and meta-analysis.Front Oncol. 2022 Jul 29;12:927510. doi: 10.3389/fonc.2022.927510. eCollection 2022. Front Oncol. 2022. PMID: 35965543 Free PMC article.
-
Mecapegfilgrastim for the prophylaxis of chemotherapy-induced neutropenia in locally advanced nasopharyngeal carcinoma: A prospective phase II clinical study.Head Neck. 2025 Jan;47(1):263-268. doi: 10.1002/hed.27897. Epub 2024 Aug 11. Head Neck. 2025. PMID: 39129235 Free PMC article. Clinical Trial.
References
-
- Joseph TSW, Tam CH, Susan LEL, et al. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer. 2010;29(5):517‐526. - PubMed
-
- Lee AWM, Tung SY, Ng WT, et al. A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10‐Year outcomes for efficacy and toxicity. Cancer. 2017;123(21):4147‐4157. - PubMed
-
- Qiu WZ, Huang PY, Shi JL, et al. Neoadjuvant chemotherapy plus intensity‐modulated radiotherapy versus concurrent chemoradiotherapy plus adjuvant chemotherapy for the treatment of locoregionally advanced nasopharyngeal carcinoma: A retrospective controlled study. Chinese Journal of Cancer. 2016;35:2. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

