Pertuzumab Plus High-Dose Trastuzumab in Patients With Progressive Brain Metastases and HER2-Positive Metastatic Breast Cancer: Primary Analysis of a Phase II Study
- PMID: 33945296
- PMCID: PMC8376355
- DOI: 10.1200/JCO.20.02822
Pertuzumab Plus High-Dose Trastuzumab in Patients With Progressive Brain Metastases and HER2-Positive Metastatic Breast Cancer: Primary Analysis of a Phase II Study
Abstract
Purpose: Effective therapies are needed for the treatment of patients with human epidermal growth factor receptor-2 (HER2)-positive metastatic breast cancer (MBC) with brain metastases. A trastuzumab radioisotope has been shown to localize in brain metastases of patients with HER2-positive MBC, and intracranial xenograft models have demonstrated a dose-dependent response to trastuzumab.
Methods: In the phase II PATRICIA study (ClinicalTrials.gov identifier: NCT02536339), patients with HER2-positive MBC with CNS metastases and CNS progression despite prior radiotherapy received pertuzumab plus high-dose trastuzumab (6 mg/kg weekly) until CNS or systemic disease progression or unacceptable toxicity. The primary end point was confirmed objective response rate (ORR) in the CNS per Response Assessment in Neuro-Oncology Brain Metastases criteria. Secondary end points included duration of response, clinical benefit rate (complete response plus partial response plus stable disease ≥ 4 or ≥ 6 months) in the CNS, and safety.
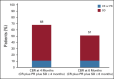
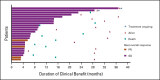
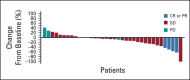
Results: Thirty-nine patients were treated for a median (range) of 4.5 (0.3-37.3) months at clinical cutoff. Thirty-seven patients discontinued treatment, most commonly because of CNS progression (n = 27); two remained on treatment. CNS ORR was 11% (95% CI, 3 to 25), with four partial responses (median duration of response, 4.6 months). Clinical benefit rate at 4 months and 6 months was 68% and 51%, respectively. Two patients permanently discontinued study treatment because of adverse events (left ventricular dysfunction [treatment-related] and seizure, both grade 3). No grade 5 adverse events were reported. No new safety signals emerged with either agent.
Conclusion: Although the CNS ORR was modest, 68% of patients experienced clinical benefit, and two patients had ongoing stable intracranial and extracranial disease for > 2 years. High-dose trastuzumab for HER2-positive CNS metastases may warrant further study.
Conflict of interest statement
Figures

Comment in
-
Letter Regarding Repeated-Measures ANOVA.J Clin Oncol. 2021 Dec 20;39(36):4127. doi: 10.1200/JCO.21.01173. Epub 2021 Oct 12. J Clin Oncol. 2021. PMID: 34637333 No abstract available.
-
Reply to J. Wei et al.J Clin Oncol. 2021 Dec 20;39(36):4127-4128. doi: 10.1200/JCO.21.01973. Epub 2021 Oct 12. J Clin Oncol. 2021. PMID: 34637335 No abstract available.
Similar articles
-
A Phase II Study of Atezolizumab, Pertuzumab, and High-Dose Trastuzumab for Central Nervous System Metastases in Patients with HER2-Positive Breast Cancer.Clin Cancer Res. 2024 Nov 1;30(21):4856-4865. doi: 10.1158/1078-0432.CCR-24-1161. Clin Cancer Res. 2024. PMID: 39226397 Free PMC article. Clinical Trial.
-
Pertuzumab plus high-dose trastuzumab for HER2-positive breast cancer with brain metastases: PATRICIA final efficacy data.NPJ Breast Cancer. 2023 Nov 17;9(1):94. doi: 10.1038/s41523-023-00587-2. NPJ Breast Cancer. 2023. PMID: 37978197 Free PMC article.
-
Afatinib alone or afatinib plus vinorelbine versus investigator's choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial.Lancet Oncol. 2015 Dec;16(16):1700-10. doi: 10.1016/S1470-2045(15)00373-3. Epub 2015 Nov 17. Lancet Oncol. 2015. PMID: 26596672 Clinical Trial.
-
Safety and Efficacy of Anti-Human Epidermal Growth Factor 2 Agents in the Treatment of Biliary Tract Cancers: A Systematic Review.JCO Precis Oncol. 2025 Apr;9:e2400594. doi: 10.1200/PO-24-00594. Epub 2025 Apr 16. JCO Precis Oncol. 2025. PMID: 40239136
-
HER2-Positive Breast Cancer-Current Treatment Management and New Therapeutic Methods for Brain Metastasis.Biomedicines. 2025 May 9;13(5):1153. doi: 10.3390/biomedicines13051153. Biomedicines. 2025. PMID: 40426980 Free PMC article. Review.
Cited by
-
Treatment options for patients with human epidermal growth factor 2-positive breast cancer brain metastases: A systematic review and meta-analysis.Front Oncol. 2023 Feb 20;13:1003565. doi: 10.3389/fonc.2023.1003565. eCollection 2023. Front Oncol. 2023. PMID: 36890831 Free PMC article.
-
Pyrotinib and trastuzumab plus palbociclib and fulvestrant in HR+/HER2+ breast cancer patients with brain metastasis.NPJ Breast Cancer. 2024 Jun 13;10(1):45. doi: 10.1038/s41523-024-00646-2. NPJ Breast Cancer. 2024. PMID: 38871705 Free PMC article.
-
Precision radiotherapy with molecular-profiling of CNS tumours.J Neurooncol. 2025 Mar;172(1):51-75. doi: 10.1007/s11060-024-04911-z. Epub 2024 Dec 19. J Neurooncol. 2025. PMID: 39699761 Review.
-
Long-term response to sequential anti-HER2 therapies including trastuzumab-deruxtecan in a patient with HER2-positive metastatic breast cancer with leptomeningeal metastases: a case report and review of the literature.Front Oncol. 2024 Jan 10;13:1210873. doi: 10.3389/fonc.2023.1210873. eCollection 2023. Front Oncol. 2024. PMID: 38269026 Free PMC article. Review.
-
Combination of tucatinib and neural stem cells secreting anti-HER2 antibody prolongs survival of mice with metastatic brain cancer.Proc Natl Acad Sci U S A. 2022 Jan 4;119(1):e2112491119. doi: 10.1073/pnas.2112491119. Proc Natl Acad Sci U S A. 2022. PMID: 34969858 Free PMC article.
References
-
- Brufsky AM Mayer M Rugo HS, et al. : Central nervous system metastases in patients with HER2-positive metastatic breast cancer: Incidence, treatment, and survival in patients from registHER. Clin Cancer Res 17:4834-4843, 2011 - PubMed
-
- Pestalozzi BC Holmes E de Azambuja E, et al. : CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: A retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol 14:244-248, 2013 - PubMed
-
- Trastuzumab (HERCEPTIN) Prescribing Information. South San Francisco, CA, Genentech, 2018
-
- Dijkers EC Oude Munnink TH Kosterink JG, et al. : Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586-592, 2010 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

