Epithelial miR-206 targets CD39/extracellular ATP to upregulate airway IL-25 and TSLP in type 2-high asthma
- PMID: 33945508
- PMCID: PMC8262281
- DOI: 10.1172/jci.insight.148103
Epithelial miR-206 targets CD39/extracellular ATP to upregulate airway IL-25 and TSLP in type 2-high asthma
Abstract
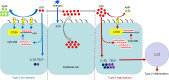
The epithelial cell-derived cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) initiate type 2 inflammation in allergic diseases, including asthma. However, the signaling pathway regulating these cytokines expression remains elusive. Since microRNAs are pivotal regulators of gene expression, we profiled microRNA expression in bronchial epithelial brushings from type 2-low and type 2-high asthma patients. miR-206 was the most highly expressed epithelial microRNA in type 2-high asthma relative to type 2-low asthma but was downregulated in both subsets compared with healthy controls. CD39, an ectonucleotidase degrading ATP, was a target of miR-206 and upregulated in asthma. Allergen-induced acute extracellular ATP accumulation led to miR-206 downregulation and CD39 upregulation in human bronchial epithelial cells, forming a feedback loop to eliminate excessive ATP. Airway ATP levels were markedly elevated and strongly correlated with IL-25 and TSLP expression in asthma patients. Intriguingly, airway miR-206 antagonism increased Cd39 expression; reduced ATP accumulation; suppressed IL-25, IL-33, and Tslp expression and group 2 innate lymphoid cell expansion; and alleviated type 2 inflammation in a mouse model of allergic airway inflammation. In contrast, airway miR-206 overexpression had opposite effects. Overall, epithelial miR-206 upregulates airway IL-25 and TSLP expression by targeting the CD39-extracellular ATP axis, which represents a potentially novel therapeutic target in type 2-high asthma.
Keywords: Asthma; Pulmonology.
Conflict of interest statement
Figures




Similar articles
-
Intelectin contributes to allergen-induced IL-25, IL-33, and TSLP expression and type 2 response in asthma and atopic dermatitis.Mucosal Immunol. 2017 Nov;10(6):1491-1503. doi: 10.1038/mi.2017.10. Epub 2017 Feb 22. Mucosal Immunol. 2017. PMID: 28224996 Free PMC article.
-
T cell-mediated induction of thymic stromal lymphopoietin in differentiated human primary bronchial epithelial cells.Clin Exp Allergy. 2014 Jul;44(7):953-64. doi: 10.1111/cea.12330. Clin Exp Allergy. 2014. PMID: 24773145
-
Effects of microRNA-19b on airway remodeling, airway inflammation and degree of oxidative stress by targeting TSLP through the Stat3 signaling pathway in a mouse model of asthma.Oncotarget. 2017 Jul 18;8(29):47533-47546. doi: 10.18632/oncotarget.17258. Oncotarget. 2017. PMID: 28472780 Free PMC article.
-
Epithelial-Derived Cytokines in Asthma.Chest. 2017 Jun;151(6):1338-1344. doi: 10.1016/j.chest.2016.10.042. Epub 2016 Nov 3. Chest. 2017. PMID: 27818325 Review.
-
Biologics and the lung: TSLP and other epithelial cell-derived cytokines in asthma.Pharmacol Ther. 2017 Jan;169:104-112. doi: 10.1016/j.pharmthera.2016.06.009. Epub 2016 Jun 27. Pharmacol Ther. 2017. PMID: 27365223 Review.
Cited by
-
Airway epithelial-derived exosomes induce acute asthma exacerbation after respiratory syncytial virus infection.MedComm (2020). 2024 Jun 27;5(7):e621. doi: 10.1002/mco2.621. eCollection 2024 Jul. MedComm (2020). 2024. PMID: 38938285 Free PMC article.
-
Exosomal microRNA/miRNA Dysregulation in Respiratory Diseases: From Mycoplasma-Induced Respiratory Disease to COVID-19 and Beyond.Cells. 2023 Oct 9;12(19):2421. doi: 10.3390/cells12192421. Cells. 2023. PMID: 37830635 Free PMC article. Review.
-
The Role of Alarmins in the Pathogenesis of Asthma.Biomolecules. 2025 Jul 11;15(7):996. doi: 10.3390/biom15070996. Biomolecules. 2025. PMID: 40723867 Free PMC article. Review.
-
Recent miRNA Research in Asthma.Curr Allergy Asthma Rep. 2022 Dec;22(12):231-258. doi: 10.1007/s11882-022-01050-1. Epub 2022 Dec 2. Curr Allergy Asthma Rep. 2022. PMID: 36459329 Free PMC article. Review.
-
Phenotype overlap in the natural history of asthma.Eur Respir Rev. 2023 May 17;32(168):220201. doi: 10.1183/16000617.0201-2022. Print 2023 Jun 30. Eur Respir Rev. 2023. PMID: 37197769 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

