Molecular signatures of labor and nonlabor myometrium with parsimonious classification from 2 calcium transporter genes
- PMID: 33945511
- PMCID: PMC8262336
- DOI: 10.1172/jci.insight.148425
Molecular signatures of labor and nonlabor myometrium with parsimonious classification from 2 calcium transporter genes
Abstract
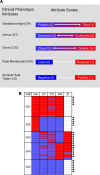
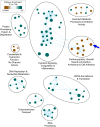
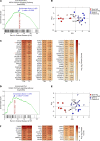
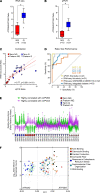
Clinical phenotyping of term and preterm labor is imprecise, and disagreement persists on categorization relative to underlying pathobiology, which remains poorly understood. We performed RNA sequencing (RNA-seq) of 31 specimens of human uterine myometrium from 10 term and 21 preterm cesarean deliveries with rich clinical context information. A molecular signature of 4814 transcripts stratified myometrial samples into quiescent (Q) and nonquiescent (NQ) phenotypes, independent of gestational age and incision site. Similar stratifications were achieved using expressed genes in Ca2+ signaling and TGF-β pathways. For maximal parsimony, we evaluated the expression of just 2 Ca2+ transporter genes, ATP2B4 (encoding PMCA4) and ATP2A2 (coding for SERCA2), and we found that their ratio reliably distinguished NQ and Q specimens in the current study, and also in 2 publicly available RNA-seq data sets (GSE50599 and GSE80172), with an overall AUC of 0.94. Cross-validation of the ATP2B4/ATP2A2 ratio by quantitative PCR in an expanded cohort (by 11 additional specimens) achieved complete separation (AUC of 1.00) of NQ versus Q specimens. While providing additional insight into the associations between clinical features of term and preterm labor and myometrial gene expression, our study also offers a practical algorithm for unbiased classification of myometrial biopsies by their overall contractile program.
Keywords: Expression profiling; Reproductive Biology.
Conflict of interest statement
Figures

Similar articles
-
Preterm labor is a distinct process from term labor following computational analysis of human myometrium.Am J Obstet Gynecol. 2022 Jan;226(1):106.e1-106.e16. doi: 10.1016/j.ajog.2021.07.002. Epub 2021 Jul 7. Am J Obstet Gynecol. 2022. PMID: 34245680
-
Integrated microRNA and mRNA network analysis of the human myometrial transcriptome in the transition from quiescence to labor.Biol Reprod. 2018 Jun 1;98(6):834-845. doi: 10.1093/biolre/ioy040. Biol Reprod. 2018. PMID: 29447339 Free PMC article.
-
Preterm labor with and without chorioamnionitis is associated with activation of myometrial inflammatory networks: a comprehensive transcriptomic analysis.Am J Obstet Gynecol. 2023 Mar;228(3):330.e1-330.e18. doi: 10.1016/j.ajog.2022.08.036. Epub 2022 Aug 21. Am J Obstet Gynecol. 2023. PMID: 36002050
-
A role of stretch-activated potassium currents in the regulation of uterine smooth muscle contraction.Acta Pharmacol Sin. 2011 Jun;32(6):758-64. doi: 10.1038/aps.2011.62. Acta Pharmacol Sin. 2011. PMID: 21642947 Free PMC article. Review.
-
Biochemistry and pharmacology of the myometrium and labor: regulation at the cellular and molecular levels.Am J Obstet Gynecol. 1982 Jan 15;142(2):225-37. doi: 10.1016/s0002-9378(16)32341-9. Am J Obstet Gynecol. 1982. PMID: 6119905 Review.
Cited by
-
LncRNA GAS5 Regulates Myometrial Cell Contractions in an m6A-Dependent Manner.Function (Oxf). 2025 Mar 24;6(2):zqaf009. doi: 10.1093/function/zqaf009. Function (Oxf). 2025. PMID: 40053491 Free PMC article.
-
Integrative analysis of noncoding mutations identifies the druggable genome in preterm birth.Sci Adv. 2024 Jan 19;10(3):eadk1057. doi: 10.1126/sciadv.adk1057. Epub 2024 Jan 19. Sci Adv. 2024. PMID: 38241369 Free PMC article.
-
KDM6B-dependent epigenetic programming of uterine fibroblasts in early pregnancy regulates parturition timing in mice.Cell. 2025 Mar 6;188(5):1265-1279.e18. doi: 10.1016/j.cell.2024.12.019. Epub 2025 Jan 21. Cell. 2025. PMID: 39842437
-
Characterization of the Myometrial Transcriptome of Long Non-coding RNA Genes in Human Labor by High-Throughput RNA-seq.Reprod Sci. 2022 Oct;29(10):2885-2893. doi: 10.1007/s43032-022-00910-5. Epub 2022 Apr 25. Reprod Sci. 2022. PMID: 35467262 Free PMC article.
-
Time Course Analysis of Transcriptome in Human Myometrium Depending on Labor Duration and Correlating With Postpartum Blood Loss.Front Genet. 2022 Jun 28;13:812105. doi: 10.3389/fgene.2022.812105. eCollection 2022. Front Genet. 2022. PMID: 35836580 Free PMC article.
References
-
- Norwitz ER, et al. Physiology of parturition. In: Resnik R, Lockwood CJ, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice, 8th ed. Elsevier; 2019:81–95.
-
- Kuba K, Bernstein PS. ACOG practice bulletin no. 188: prelabor rupture of membranes. Obstet Gynecol. 2018;131(6):1163–1164. - PubMed
-
- Simhan HN, et al. Prevention and management of preterm parturition. In: Resnik R, Lockwood CJ, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice, 8th ed. Elsevier; 2019:679–711.
-
- Buhimschi CS, et al. Pathogenesis of spontaneous preterm birth. In: Resnik R, Lockwood CJ, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice, 8th ed. Elsevier; 2019:96–126.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

