SPATS1 (spermatogenesis-associated, serine-rich 1) is not essential for spermatogenesis and fertility in mouse
- PMID: 33945571
- PMCID: PMC8096103
- DOI: 10.1371/journal.pone.0251028
SPATS1 (spermatogenesis-associated, serine-rich 1) is not essential for spermatogenesis and fertility in mouse
Abstract
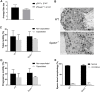
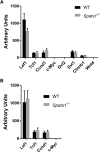
SPATS1 (spermatogenesis-associated, serine-rich 1) is an evolutionarily conserved, testis-specific protein that is differentially expressed during rat male meiotic prophase. Some reports have suggested a link between SPATS1 underexpression/mutation and human pathologies such as male infertility and testicular cancer. Given the absence of functional studies, we generated a Spats1 loss-of-function mouse model using CRISPR/Cas9 technology. The phenotypic analysis showed no overt phenotype in Spats1-/- mice, with both males and females being fertile. Flow cytometry and histological analyses did not show differences in the testicular content and histology between WT and knockout mice. Moreover, no significant differences in sperm concentration, motility, and morphology, were observed between WT and KO mice. These results were obtained both for young adults and for aged animals. Besides, although an involvement of SPATS1 in the Wnt signaling pathway has been suggested, we did not detect changes in the expression levels of typical Wnt pathway-target genes in mutant individuals. Thus, albeit Spats1 alteration might be a risk factor for male testicular health, we hereby show that this gene is not individually essential for male fertility and spermatogenesis in mouse.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Testis-specific serine kinase 3 is required for sperm morphogenesis and male fertility.Andrology. 2023 Jul;11(5):826-839. doi: 10.1111/andr.13314. Epub 2022 Nov 16. Andrology. 2023. PMID: 36306217 Free PMC article.
-
Germ cell-specific disruption of the Meig1 gene causes impaired spermiogenesis in mice.Andrology. 2013 Jan;1(1):37-46. doi: 10.1111/j.2047-2927.2012.00001.x. Epub 2012 Aug 30. Andrology. 2013. PMID: 23258628 Free PMC article.
-
The testis-specifically expressed Dpep3 is not essential for male fertility in mice.Gene. 2019 Aug 30;711:143925. doi: 10.1016/j.gene.2019.06.015. Epub 2019 Jun 15. Gene. 2019. PMID: 31212048
-
Testis-specific serine kinase protein family in male fertility and as targets for non-hormonal male contraception†.Biol Reprod. 2020 Aug 4;103(2):264-274. doi: 10.1093/biolre/ioaa064. Biol Reprod. 2020. PMID: 32337545 Free PMC article. Review.
-
Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice.Endocr Rev. 2009 Apr;30(2):119-32. doi: 10.1210/er.2008-0025. Epub 2009 Jan 27. Endocr Rev. 2009. PMID: 19176467 Free PMC article. Review.
Cited by
-
Expression and Characterization of the Spats1 Gene and Its Response to E2/MT Treatment in the Chinese Soft-Shelled Turtle (Pelodiscus sinensis).Animals (Basel). 2022 Jul 21;12(14):1858. doi: 10.3390/ani12141858. Animals (Basel). 2022. PMID: 35883403 Free PMC article.
-
lncRNA 1700101O22Rik and NONMMUG030480.1 Are Not Essential for Spermatogenesis in Mice.Int J Mol Sci. 2022 Aug 3;23(15):8627. doi: 10.3390/ijms23158627. Int J Mol Sci. 2022. PMID: 35955762 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

