Evaluation of potential COVID-19 recurrence in patients with late repeat positive SARS-CoV-2 testing
- PMID: 33945583
- PMCID: PMC8096096
- DOI: 10.1371/journal.pone.0251214
Evaluation of potential COVID-19 recurrence in patients with late repeat positive SARS-CoV-2 testing
Abstract
Background: SARS-CoV-2 reinfection and reactivation has mostly been described in case reports. We therefore investigated the epidemiology of recurrent COVID-19 SARS-CoV-2.
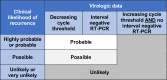
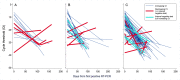
Methods: Among patients testing positive for SARS-CoV-2 between March 11 and July 31, 2020 within an integrated healthcare system, we identified patients with a recurrent positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) assay ≥60 days after an initial positive test. To assign an overall likelihood of COVID-19 recurrence, we combined quantitative data from initial and recurrent positive RT-PCR cycle thresholds-a value inversely correlated with viral RNA burden- with a clinical recurrence likelihood assigned based on independent, standardized case review by two physicians. "Probable" or "possible" recurrence by clinical assessment was confirmed as the final recurrence likelihood only if a cycle threshold value obtained ≥60 days after initial testing was lower than its preceding cycle threshold or if the patient had an interval negative RT-PCR.
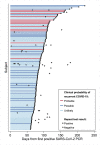
Results: Among 23,176 patients testing positive for SARS-CoV-2, 1,301 (5.6%) had at least one additional SARS-CoV-2 RT-PCRs assay ≥60 days later. Of 122 testing positive, 114 had sufficient data for evaluation. The median interval to the recurrent positive RT-PCR was 85.5 (IQR 74-107) days. After combining clinical and RT-PCR cycle threshold data, four patients (3.5%) met criteria for probable COVID-19 recurrence. All four exhibited symptoms at recurrence and three required a higher level of medical care compared to their initial diagnosis. After including six additional patients (5.3%) with possible recurrence, recurrence incidence was 4.3 (95% CI 2.1-7.9) cases per 10,000 COVID-19 patients.
Conclusions: Only 0.04% of all COVID-19 patients in our health system experienced probable or possible recurrence; 90% of repeat positive SARS-CoV-2 RT-PCRs were not consistent with true recurrence. Our pragmatic approach combining clinical and quantitative RT-PCR data could aid assessment of COVID-19 reinfection or reactivation by clinicians and public health personnel.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Dr. Peltan reports research support from the National Institutes of Health, Centers for Disease Control and Janssen and funding to his institution from Asahi Kasei Pharma and Regeneron. Dr. Brown reports personal fees from New York University, Hamilton, Oxford University Press and research support from the National Institutes of Health, the Department of Defense, Faron, Sedana Medical, and Janssen. Dr. Lopansri reports personal fees from Premier Inc., and Luminex and research support from Immunexpress Inc. and Opgen. Dr. Webb reports grant support from the Agency for Healthcare Research and Quality and funding to his institution from Abbvie, Genentech, Gilead, Regeneron, Roche, and the U.S. National Institutes of Health. This does not alter our adherence to PLOS ONE policies on sharing data and materials. The authors otherwise have declared that no competing interests exist.
Figures

References
-
- Findings from investigation and analysis of re-positive cases. In: Korea CDC [Internet]. 19 May 2020 [cited 25 Oct 2020]. Available: https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

