A PSGL-1 glycomimetic reduces thrombus burden without affecting hemostasis
- PMID: 33945603
- PMCID: PMC8570056
- DOI: 10.1182/blood.2020009428
A PSGL-1 glycomimetic reduces thrombus burden without affecting hemostasis
Abstract
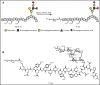
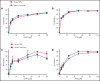
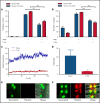
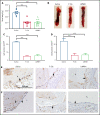
Events mediated by the P-selectin/PSGL-1 pathway play a critical role in the initiation and propagation of venous thrombosis by facilitating the accumulation of leukocytes and platelets within the growing thrombus. Activated platelets and endothelium express P-selectin, which binds P-selectin glycoprotein ligand-1 (PSGL-1) that is expressed on the surface of all leukocytes. We developed a pegylated glycomimetic of the N terminus of PSGL-1, PEG40-GSnP-6 (P-G6), which proved to be a highly potent P-selectin inhibitor with a favorable pharmacokinetic profile for clinical translation. P-G6 inhibits human and mouse platelet-monocyte and platelet-neutrophil aggregation in vitro and blocks microcirculatory platelet-leukocyte interactions in vivo. Administration of P-G6 reduces thrombus formation in a nonocclusive model of deep vein thrombosis with a commensurate reduction in leukocyte accumulation, but without disruption of hemostasis. P-G6 potently inhibits the P-selectin/PSGL-1 pathway and represents a promising drug candidate for the prevention of venous thrombosis without increased bleeding risk.
© 2021 by The American Society of Hematology.
Figures


Comment in
-
A long-half-life, high-affinity P-selectin inhibitor.Blood. 2021 Sep 30;138(13):1096-1097. doi: 10.1182/blood.2021012302. Blood. 2021. PMID: 34591097 No abstract available.
References
-
- Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. - PubMed
-
- Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495-S501. - PubMed
-
- Kearon C, Akl EA, Ornelas J, et al. . Antithrombotic therapy for VTE disease: Chest guideline and expert panel report [published correction appears in Chest. 2016;150(4):988]. Chest. 2016;149(2):315-352. - PubMed
-
- Carrier M, Abou-Nassar K, Mallick R, et al. ; AVERT Investigators . Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711-719. - PubMed
-
- Coulis AA, Mackey WC. A review of the efficacy and safety profiles of the novel oral anticoagulants in the treatment and prevention of venous thromboembolism. Clin Ther. 2018;40(12):2140-2167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

