BCOR gene alterations in hematologic diseases
- PMID: 33945606
- PMCID: PMC8887995
- DOI: 10.1182/blood.2021010958
BCOR gene alterations in hematologic diseases
Abstract
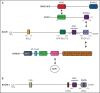
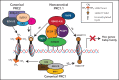
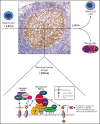
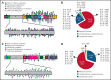
The BCL6 corepressor (BCOR) is a transcription factor involved in the control of embryogenesis, mesenchymal stem cells function, hematopoiesis, and lymphoid development. Recurrent somatic clonal mutations of the BCOR gene and its homolog BCORL1 have been detected in several hematologic malignancies and aplastic anemia. They are scattered across the whole gene length and mostly represent frameshifts (deletions, insertions), nonsense, and missence mutations. These disruptive events lead to the loss of full-length BCOR protein and to the lack or low expression of a truncated form of the protein, both consistent with the tumor suppressor role of BCOR.BCOR and BCORL1 mutations are similar to those causing 2 rare X-linked diseases: oculofaciocardiodental (OFCD) and Shukla-Vernon syndromes, respectively. Here, we focus on the structure and function of normal BCOR and BCORL1 in normal hematopoietic and lymphoid tissues and review the frequency and clinical significance of the mutations of these genes in malignant and nonmalignant hematologic diseases. Moreover, we discuss the importance of mouse models to better understand the role of Bcor loss, alone and combined with alterations of other genes (eg, Dnmt3a and Tet2), in promoting hematologic malignancies and in providing a useful platform for the development of new targeted therapies.
© 2021 by The American Society of Hematology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

