iPSC-derived myelinoids to study myelin biology of humans
- PMID: 33945785
- PMCID: PMC8098746
- DOI: 10.1016/j.devcel.2021.04.006
iPSC-derived myelinoids to study myelin biology of humans
Erratum in
-
iPSC-derived myelinoids to study myelin biology of humans.Dev Cell. 2022 Jan 10;57(1):146. doi: 10.1016/j.devcel.2021.12.009. Dev Cell. 2022. PMID: 35016003 Free PMC article. No abstract available.
Abstract
Myelination is essential for central nervous system (CNS) formation, health, and function. Emerging evidence of oligodendrocyte heterogeneity in health and disease and divergent CNS gene expression profiles between mice and humans supports the development of experimentally tractable human myelination systems. Here, we developed human iPSC-derived myelinating organoids ("myelinoids") and quantitative tools to study myelination from oligodendrogenesis through to compact myelin formation and myelinated axon organization. Using patient-derived cells, we modeled a monogenetic disease of myelinated axons (Nfasc155 deficiency), recapitulating impaired paranodal axo-glial junction formation. We also validated the use of myelinoids for pharmacological assessment of myelination-both at the level of individual oligodendrocytes and globally across whole myelinoids-and demonstrated reduced myelination in response to suppressed synaptic vesicle release. Our study provides a platform to investigate human myelin development, disease, and adaptive myelination.
Keywords: adaptive myelination; disease modelling; human myelination; human stem cell; iPSC; myelin; myelinoid; oligodendrocyte; organoid; paranode.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.C. is a co-founder of Pheno Therapeutics. All other authors declare no competing interests.
Figures

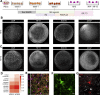
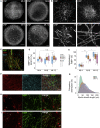

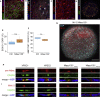
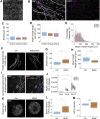
Comment in
-
Modeling myelin: A toolkit for exploring myelin's mysteries in vitro.Dev Cell. 2021 May 3;56(9):1215-1217. doi: 10.1016/j.devcel.2021.04.015. Dev Cell. 2021. PMID: 33945781
Similar articles
-
Human IPSC-Derived Model to Study Myelin Disruption.Int J Mol Sci. 2021 Aug 31;22(17):9473. doi: 10.3390/ijms22179473. Int J Mol Sci. 2021. PMID: 34502381 Free PMC article.
-
Double myelination of axons in the sympathetic nervous system of the mouse. II. Mechanisms of formation.J Neurocytol. 1988 Apr;17(2):263-76. doi: 10.1007/BF01674212. J Neurocytol. 1988. PMID: 3204414
-
Aligned Brain Extracellular Matrix Promotes Differentiation and Myelination of Human-Induced Pluripotent Stem Cell-Derived Oligodendrocytes.ACS Appl Mater Interfaces. 2019 May 1;11(17):15344-15353. doi: 10.1021/acsami.9b03242. Epub 2019 Apr 22. ACS Appl Mater Interfaces. 2019. PMID: 30974942
-
Myelinated nerve fibres in the CNS.Prog Neurobiol. 1993 Mar;40(3):319-84. doi: 10.1016/0301-0082(93)90015-k. Prog Neurobiol. 1993. PMID: 8441812 Review.
-
On the resemblance of synapse formation and CNS myelination.Neuroscience. 2014 Sep 12;276:98-108. doi: 10.1016/j.neuroscience.2013.08.062. Epub 2013 Sep 12. Neuroscience. 2014. PMID: 24035825 Review.
Cited by
-
Human-Based New Approach Methodologies in Developmental Toxicity Testing: A Step Ahead from the State of the Art with a Feto-Placental Organ-on-Chip Platform.Int J Environ Res Public Health. 2022 Nov 28;19(23):15828. doi: 10.3390/ijerph192315828. Int J Environ Res Public Health. 2022. PMID: 36497907 Free PMC article. Review.
-
Small-molecule-induced epigenetic rejuvenation promotes SREBP condensation and overcomes barriers to CNS myelin regeneration.Cell. 2024 May 9;187(10):2465-2484.e22. doi: 10.1016/j.cell.2024.04.005. Epub 2024 May 2. Cell. 2024. PMID: 38701782 Free PMC article.
-
The ciliary gene INPP5E confers dorsal telencephalic identity to human cortical organoids by negatively regulating Sonic hedgehog signaling.Cell Rep. 2022 May 17;39(7):110811. doi: 10.1016/j.celrep.2022.110811. Cell Rep. 2022. PMID: 35584663 Free PMC article.
-
Fast generation of forebrain oligodendrocyte spheroids from human embryonic stem cells by transcription factors.iScience. 2022 Sep 20;25(10):105172. doi: 10.1016/j.isci.2022.105172. eCollection 2022 Oct 21. iScience. 2022. PMID: 36217550 Free PMC article.
-
Transactive response DNA-binding protein-43 proteinopathy in oligodendrocytes revealed using an induced pluripotent stem cell model.Brain Commun. 2021 Oct 26;3(4):fcab255. doi: 10.1093/braincomms/fcab255. eCollection 2021. Brain Commun. 2021. PMID: 35350711 Free PMC article.
References
-
- Aarts E., Verhage M., Veenvliet J.V., Dolan C.V., van der Sluis S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat. Neurosci. 2014;17:491–496. - PubMed
-
- Ameis S.H., Catani M. Altered white matter connectivity as a neural substrate for social impairment in autism spectrum disorder. Cortex. 2015;62:158–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

