Long-term efficacy and safety of fostemsavir among subgroups of heavily treatment-experienced adults with HIV-1
- PMID: 33946085
- PMCID: PMC8183480
- DOI: 10.1097/QAD.0000000000002851
Long-term efficacy and safety of fostemsavir among subgroups of heavily treatment-experienced adults with HIV-1
Abstract
Objectives: The aim of this study was to understand how demographic and treatment-related factors impact responses to fostemsavir-based regimens.
Design: BRIGHTE is an ongoing phase 3 study evaluating twice-daily fostemsavir 600 mg and optimized background therapy (OBT) in heavily treatment-experienced individuals failing antiretroviral therapy with limited treatment options (Randomized Cohort 1-2 and Nonrandomized Cohort 0 fully active antiretroviral classes).
Methods: Virologic response rates (HIV-1 RNA <40 copies/ml, Snapshot analysis) and CD4+ T-cell count increases in the Randomized Cohort were analysed by prespecified baseline characteristics (age, race, sex, region, HIV-1 RNA, CD4+ T-cell count) and viral susceptibility to OBT. Safety results were analysed by baseline characteristics for combined cohorts (post hoc).
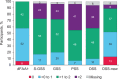
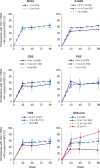
Results: In the Randomized Cohort, virologic response rates increased between Weeks 24 and 96 across most subgroups. Virologic response rates over time were most clearly associated with overall susceptibility scores for new OBT agents (OSS-new). CD4+ T-cell count increases were comparable across subgroups. Participants with baseline CD4+ T-cell counts less than 20 cells/μl had a mean increase of 240 cells/μl. In the safety population, more participants with baseline CD4+ T-cell counts less than 20 vs. at least 200 cells/μl had grade 3/4 adverse events [53/107 (50%) vs. 24/96 (25%)], serious adverse events [58/107 (54%) vs. 25/96 (26%)] and deaths [16/107 (15%) vs. 2/96 (2%)]. There were no safety differences by other subgroups.
Conclusion: Week 96 results for BRIGHTE demonstrate comparable rates of virologic and immunologic response (Randomized Cohort) and safety (combined cohorts) across subgroups. OSS-new is an important consideration when constructing optimized antiretroviral regimens for heavily treatment-experienced individuals with limited remaining treatment options.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
P.A., S.C., A.P., C.L. and M.L. are employees of ViiV Healthcare and hold stock in GlaxoSmithKline. M.T. has participated in clinical trials for ViiV Healthcare, Bristol-Myers Squibb, Cepheid, Cytodyn, Gilead, GlaxoSmithKline, Merck Sharp and Dohme, and Frontier Biotechnologies through AIDS Research Consortium of Atlanta. J.-M.M. has received honoraria for advisory board participation from ViiV Healthcare, Gilead, Merck and Sanofi and a grant from Gilead. J.A. has participated in clinical trials for Atea, Bristol-Myers Squibb, ViiV Healthcare, Gilead, Janssen, Merck, Pfizer, Regeneron, Frontier Technology and Shionogi, for which her institution received grants, and has received personal fees for scientific advisory board participation from Gilead, Merck, Janssen, Medicure, Theratechnologies and ViiV Healthcare. I.C. has served as the principal investigator for studies funded by ViiV Healthcare, Merck and Janssen, for which her institution received grants, and has received honoraria for advisory board participation from ViiV Healthcare, Merck and Janssen. M.K. has served as the principal investigator for studies funded by ViiV Healthcare and Gilead, for which his institution has received grants. M.R. has received speaking and/or consulting fees from Gilead, ViiV Healthcare, Janssen and Merck. E.S. has received grants and/or personal fees for advisory board participation from GlaxoSmithKline, Janssen, Merck, Pfizer and Gilead. G.P. has received grants and/or personal fees from Gilead, Nephrotek, ViiV Healthcare, AbbVie, Merck Sharp and Dohme, Bristol-Myers Squibb and Boehringer Ingelheim. P.N.K. has received grants and/or personal fees for advisory board participation from GlaxoSmithKline, Merck and Theratechnologies and holds stock in GlaxoSmithKline, Pfizer, Johnson and Johnson, Merck and Gilead. M.W. is an employee of and holds stock in GlaxoSmithKline. A.C., M.M., S.T-P., A.S-C. and G.H.L. have no conflicts to disclose. This study was funded by ViiV Healthcare.
M.T., J.-M.M., J.A., M.K., C.L. and M.L. contributed to the conception of the study. M.K., C.L. and M.L. contributed to the design of the study. M.T., J.-M.M., J.A., I.C., M.K., A.C., M.M., M.R., E.S., S.T.-P., A.S.-C., G.H.L., G.P. and P.N.K. contributed to the acquisition of data. P.A., M.W., S.C., A.P., C.L. and M.L. contributed to the analysis of data. All authors contributed to the interpretation of data, drafting the manuscript, and critically revising the manuscript for important intellectual content. All authors approve the manuscript for publication.
Anonymized individual participant data and study documents can be requested for further research from
Figures

References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Ad.... [Accessed 10 January 2020].
-
- Pelchen-Matthews A, Borges AH, Reekie J, Rasmussen LD, Wiese L, Weber J, et al.Prevalence and outcomes for heavily treatment-experienced (HTE) individuals living with HIV in a European cohort. [Abstract TUPEB22] 10th IAS Conference on HIV Science (IAS); Mexico, City, Mexico, 21–24 July 2019.
-
- European AIDS Clinical Society. Guidelines version 10.0: November 2019. https://www.eacsociety.org/files/2019_guidelines-10.0_final.pdf. [Accessed 1 February 2020].
-
- Armenia D, Di Carlo D, Flandre P, Bouba Y, Borghi V, Forbici F, et al. . HIV MDR is still a relevant issue despite its dramatic drop over the years. J Antimicrob Chemother 2020; 75:1301–1310. - PubMed
-
- Henegar C, Vannappagari V, Viswanathan S, DeKoven M, Clark A, Ackerman P, et al. . Identifying heavily treatment-experienced patients in a large administrative claims database. [Abstract MOPEB236] 10th IAS Conference on HIV Science (IAS); Mexico, City, Mexico, 21–24 July 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

