Efficacy and Pharmacological Appropriateness of Cinnarizine and Dimenhydrinate in the Treatment of Vertigo and Related Symptoms
- PMID: 33946152
- PMCID: PMC8125582
- DOI: 10.3390/ijerph18094787
Efficacy and Pharmacological Appropriateness of Cinnarizine and Dimenhydrinate in the Treatment of Vertigo and Related Symptoms
Abstract
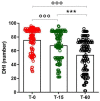
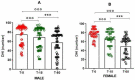
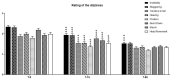
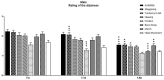
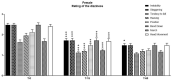
Vertigo is not itself a disease, but rather a symptom of various syndromes and disorders that jeopardize balance function, which is essential for daily activities. It is an abnormal sensation of motion that usually occurs in the absence of motion, or when a motion is sensed inaccurately. Due to the complexity of the etiopathogenesis of vertigo, many pharmacological treatments have been tested for efficacy on vertigo. Among these drugs, cinnarizine, usually given together with dimenhydrinate, appears to be the first-line pharmacotherapy for the management of vertigo and inner ear disorders. Based on these considerations, the present non-interventional study aimed to investigate the clinical efficacy and tolerability of a fixed combination of cinnarizine (20 mg) and dimenhydrinate (40 mg) in patients suffering from vertigo-related symptoms. To this end, we enrolled 120 adults-70 males, and 50 females-with an average age of 64 years. Before beginning pharmacological treatment, all patients were screened for the intensity of vertigo, dizziness, and concomitant symptoms through the Visual Scale of Dizziness Disorders and Dizziness Handicap Inventory scales. At the end of the anamnestic evaluation, patients received the fixed-dose combination of cinnarizine (20 mg) plus dimenhydrinate (40 mg) 3 times daily, for 60 days. The results of this study provide further insight regarding the efficacy of the fixed combination when used to reduce symptoms of vestibular vertigo of central and/or peripheral origin, after both the 15- and 60-day therapies. Independent of the type of vertigo, the fixed combination was able to reduce dizziness- and vertigo-associated symptoms in more than 75% of all patients treated, starting from 15 days of therapy, and improving 60 days after starting the therapy. Interestingly, we also found differences between male and female patients in the framework of the pharmacological effects of therapy. This study provides further details concerning the therapeutic efficacy of the fixed combination of cinnarizine and dimenhydrinate, and also focuses attention on the possibility that these drugs could act in a gender-specific manner, paving the way for further research.
Keywords: cinnarizine; dimenhydrinate; dizziness; pharmacological treatment of vertigo; vertigo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cirek Z., Schwarz M., Baumann W., Novotny M. Efficacy and Tolerability of a FixedCombination of Cinnarizine and Dimenhydrinate versus Betahistine in the Treatment of Otogenic Vertigo: A Double-Blind, Randomised Clinical Study. Clin. Drug. Investig. 2005;25:377–389. doi: 10.2165/00044011-200525060-00003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

