Utility of Routine Laboratory Biomarkers to Detect COVID-19: A Systematic Review and Meta-Analysis
- PMID: 33946171
- PMCID: PMC8147047
- DOI: 10.3390/v13050803
Utility of Routine Laboratory Biomarkers to Detect COVID-19: A Systematic Review and Meta-Analysis
Abstract
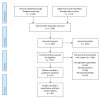
No routine laboratory biomarkers perform well enough in diagnosing COVID-19 in isolation for them to be used as a standalone diagnostic test or to help clinicians prioritize patients for treatment. Instead, other diagnostic tests are needed. The aim of this work was to statistically summarise routine laboratory biomarker measurements in COVID-19-positive and -negative patients to inform future work. A systematic literature review and meta-analysis were performed. The search included names of commonly used, routine laboratory tests in the UK NHS, and focused on research papers reporting laboratory results of patients diagnosed with COVID-19. A random effects meta-analysis of the standardized mean difference between COVID-19-positive and -negative groups was conducted for each biomarker. When comparing reported laboratory biomarker results, we identified decreased white blood cell, neutrophil, lymphocyte, eosinophil, and platelet counts; while lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase were elevated in COVID-19-positive compared to COVID-19-negative patients. Differences were identified across a number of routine laboratory biomarkers between COVID-19-positive and -negative patients. Further research is required to identify whether routine laboratory biomarkers can be used in the development of a clinical scoring system to aid with triage of patients.
Keywords: COVID-19; biomarker; diagnosis; meta-analysis; systematic review.
Conflict of interest statement
The authors declare no conflict of interest.
References
-
- WHO Timeline: WHO’s COVID-19 Response. [(accessed on 23 September 2020)]; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interact....
-
- PHE, COVID-19: Guidance for Sampling and for Diagnostic Laboratories. [(accessed on 29 July 2020)]; Available online: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-guida....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical