Plant Acyl-CoA-Binding Proteins-Their Lipid and Protein Interactors in Abiotic and Biotic Stresses
- PMID: 33946260
- PMCID: PMC8146436
- DOI: 10.3390/cells10051064
Plant Acyl-CoA-Binding Proteins-Their Lipid and Protein Interactors in Abiotic and Biotic Stresses
Abstract
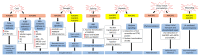
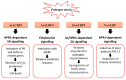
Plants are constantly exposed to environmental stresses during their growth and development. Owing to their immobility, plants possess stress-sensing abilities and adaptive responses to cope with the abiotic and biotic stresses caused by extreme temperatures, drought, flooding, salinity, heavy metals and pathogens. Acyl-CoA-binding proteins (ACBPs), a family of conserved proteins among prokaryotes and eukaryotes, bind to a variety of acyl-CoA esters with different affinities and play a role in the transport and maintenance of subcellular acyl-CoA pools. In plants, studies have revealed ACBP functions in development and stress responses through their interactions with lipids and protein partners. This review summarises the roles of plant ACBPs and their lipid and protein interactors in abiotic and biotic stress responses.
Keywords: abiotic stress; acyl-CoA-binding proteins; biotic stress; lipids; protein interactors; stress signalling.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
-
- Huang A.H.C. Oil bodies and oleosins in seeds. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992;43:177–200. doi: 10.1146/annurev.pp.43.060192.001141. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- The Wilson and Amelia Wong Endowment Fund
- AoE/M-403/16 and AoE/M-05/12/Hong Kong Research Grants Council Area of Excellence Schemes
- 17101818 and 17109917/The General Research Fund
- The Innovation Technology Fund of the Innovation Technology Commission (Funding Support to the State Key Laboratory of Agrobiotechnology in Hong Kong)
LinkOut - more resources
Full Text Sources
Other Literature Sources

