D936Y and Other Mutations in the Fusion Core of the SARS-CoV-2 Spike Protein Heptad Repeat 1: Frequency, Geographical Distribution, and Structural Effect
- PMID: 33946306
- PMCID: PMC8124767
- DOI: 10.3390/molecules26092622
D936Y and Other Mutations in the Fusion Core of the SARS-CoV-2 Spike Protein Heptad Repeat 1: Frequency, Geographical Distribution, and Structural Effect
Abstract
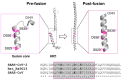
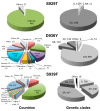
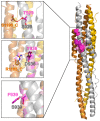
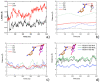
The crown of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is constituted by its spike (S) glycoprotein. S protein mediates the SARS-CoV-2 entry into the host cells. The "fusion core" of the heptad repeat 1 (HR1) on S plays a crucial role in the virus infectivity, as it is part of a key membrane fusion architecture. While SARS-CoV-2 was becoming a global threat, scientists have been accumulating data on the virus at an impressive pace, both in terms of genomic sequences and of three-dimensional structures. On 15 February 2021, from the SARS-CoV-2 genomic sequences in the GISAID resource, we collected 415,673 complete S protein sequences and identified all the mutations occurring in the HR1 fusion core. This is a 21-residue segment, which, in the post-fusion conformation of the protein, gives many strong interactions with the heptad repeat 2, bringing viral and cellular membranes in proximity for fusion. We investigated the frequency and structural effect of novel mutations accumulated over time in such a crucial region for the virus infectivity. Three mutations were quite frequent, occurring in over 0.1% of the total sequences. These were S929T, D936Y, and S949F, all in the N-terminal half of the HR1 fusion core segment and particularly spread in Europe and USA. The most frequent of them, D936Y, was present in 17% of sequences from Finland and 12% of sequences from Sweden. In the post-fusion conformation of the unmutated S protein, D936 is involved in an inter-monomer salt bridge with R1185. We investigated the effect of the D936Y mutation on the pre-fusion and post-fusion state of the protein by using molecular dynamics, showing how it especially affects the latter one.
Keywords: COVID-19; infectivity; molecular dynamics; mutations; spike protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Spike protein fusion loop controls SARS-CoV-2 fusogenicity and infectivity.J Struct Biol. 2021 Jun;213(2):107713. doi: 10.1016/j.jsb.2021.107713. Epub 2021 Mar 1. J Struct Biol. 2021. PMID: 33662570 Free PMC article.
-
Protein mimics of fusion core from SARS-CoV-1 can inhibit SARS-CoV-2 entry.Biochem Biophys Res Commun. 2024 Dec 3;736:150857. doi: 10.1016/j.bbrc.2024.150857. Epub 2024 Oct 20. Biochem Biophys Res Commun. 2024. PMID: 39490155
-
Stabilizing the closed SARS-CoV-2 spike trimer.Nat Commun. 2021 Jan 11;12(1):244. doi: 10.1038/s41467-020-20321-x. Nat Commun. 2021. PMID: 33431842 Free PMC article.
-
Structural basis of severe acute respiratory syndrome coronavirus 2 infection.Curr Opin HIV AIDS. 2021 Jan;16(1):74-81. doi: 10.1097/COH.0000000000000658. Curr Opin HIV AIDS. 2021. PMID: 33186231 Review.
-
Domains and Functions of Spike Protein in Sars-Cov-2 in the Context of Vaccine Design.Viruses. 2021 Jan 14;13(1):109. doi: 10.3390/v13010109. Viruses. 2021. PMID: 33466921 Free PMC article. Review.
Cited by
-
SARS-CoV-2 growth, furin-cleavage-site adaptation and neutralization using serum from acutely infected hospitalized COVID-19 patients.J Gen Virol. 2020 Nov;101(11):1156-1169. doi: 10.1099/jgv.0.001481. Epub 2020 Aug 21. J Gen Virol. 2020. PMID: 32821033 Free PMC article.
-
Immunoinformatics-Aided Design of a Peptide Based Multiepitope Vaccine Targeting Glycoproteins and Membrane Proteins against Monkeypox Virus.Viruses. 2022 Oct 27;14(11):2374. doi: 10.3390/v14112374. Viruses. 2022. PMID: 36366472 Free PMC article.
-
Ensemble-Based Mutational Profiling and Network Analysis of the SARS-CoV-2 Spike Omicron XBB Lineages for Interactions with the ACE2 Receptor and Antibodies: Cooperation of Binding Hotspots in Mediating Epistatic Couplings Underlies Binding Mechanism and Immune Escape.Int J Mol Sci. 2024 Apr 12;25(8):4281. doi: 10.3390/ijms25084281. Int J Mol Sci. 2024. PMID: 38673865 Free PMC article.
-
Spatio-Temporal Mutational Profile Appearances of Swedish SARS-CoV-2 during the Early Pandemic.Viruses. 2020 Sep 14;12(9):1026. doi: 10.3390/v12091026. Viruses. 2020. PMID: 32937868 Free PMC article.
-
COVID-19 Variants and Vaccine Development.Viruses. 2024 May 10;16(5):757. doi: 10.3390/v16050757. Viruses. 2024. PMID: 38793638 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

