Electrophysiological Properties of Endogenous Single Ca2+ Activated Cl- Channels Induced by Local Ca2+ Entry in HEK293
- PMID: 33946319
- PMCID: PMC8124839
- DOI: 10.3390/ijms22094767
Electrophysiological Properties of Endogenous Single Ca2+ Activated Cl- Channels Induced by Local Ca2+ Entry in HEK293
Abstract
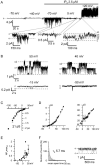
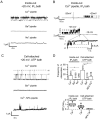
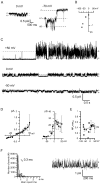
Microdomains formed by proteins of endoplasmic reticulum and plasma membrane play a key role in store-operated Ca2+ entry (SOCE). Ca2+ release through inositol 1,4,5-trisphosphate receptor (IP3R) and subsequent Ca2+ store depletion activate STIM (stromal interaction molecules) proteins, sensors of intraluminal Ca2+, which, in turn, open the Orai channels in plasma membrane. Downstream to this process could be activated TRPC (transient receptor potential-canonical) calcium permeable channels. Using single channel patch-clamp technique we found that a local Ca2+ entry through TRPC1 channels activated endogenous Ca2+-activated chloride channels (CaCCs) with properties similar to Anoctamin6 (TMEM16F). Our data suggest that their outward rectification is based on the dependence from membrane potential of both the channel conductance and the channel activity: (1) The conductance of active CaCCs highly depends on the transmembrane potential (from 3 pS at negative potentials till 60 pS at positive potentials); (2) their activity (NPo) is enhanced with increasing Ca2+ concentration and/or transmembrane potential, conversely lowering of intracellular Ca2+ concentration reduced the open state dwell time; (3) CaCC amplitude is only slightly increased by intracellular Ca2+ concentration. Experiments with Ca2+ buffering by EGTA or BAPTA suggest close local arrangement of functional CaCCs and TRPC1 channels. It is supposed that Ca2+-activated chloride channels are involved in Ca2+ entry microdomains.
Keywords: CaCC; HEK293; IP3R; SOCE; TMEM16; TRPC1; anoctamin; single channel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Intricate interaction between store-operated calcium entry and calcium-activated chloride channels in pulmonary artery smooth muscle cells.Adv Exp Med Biol. 2010;661:31-55. doi: 10.1007/978-1-60761-500-2_3. Adv Exp Med Biol. 2010. PMID: 20204722
-
TMEM16F is a component of a Ca2+-activated Cl- channel but not a volume-sensitive outwardly rectifying Cl- channel.Am J Physiol Cell Physiol. 2013 Apr 15;304(8):C748-59. doi: 10.1152/ajpcell.00228.2012. Epub 2013 Feb 20. Am J Physiol Cell Physiol. 2013. PMID: 23426967
-
STIM1 and STIM2 proteins differently regulate endogenous store-operated channels in HEK293 cells.J Biol Chem. 2015 Feb 20;290(8):4717-4727. doi: 10.1074/jbc.M114.601856. Epub 2014 Dec 22. J Biol Chem. 2015. PMID: 25533457 Free PMC article.
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
-
New Aspects of the Contribution of ER to SOCE Regulation: TRPC Proteins as a Link Between Plasma Membrane Ion Transport and Intracellular Ca2+ Stores.Adv Exp Med Biol. 2017;993:239-255. doi: 10.1007/978-3-319-57732-6_13. Adv Exp Med Biol. 2017. PMID: 28900918 Review.
Cited by
-
The Effect of Calcium Ions on the Electrophysiological Properties of Single ANO6 Channels.Acta Naturae. 2024 Jan-Mar;16(1):40-47. doi: 10.32607/actanaturae.27338. Acta Naturae. 2024. PMID: 38698960 Free PMC article.
-
NitrOFF: An engineered fluorescent biosensor to illuminate nitrate transport in living cells.bioRxiv [Preprint]. 2025 Mar 23:2025.03.22.644677. doi: 10.1101/2025.03.22.644677. bioRxiv. 2025. PMID: 40166251 Free PMC article. Preprint.
-
The tether function of the anoctamins.Cell Calcium. 2024 Jul;121:102875. doi: 10.1016/j.ceca.2024.102875. Epub 2024 Apr 20. Cell Calcium. 2024. PMID: 38701708 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

