Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism
- PMID: 33946426
- PMCID: PMC8147131
- DOI: 10.3390/cells10051067
Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism
Abstract
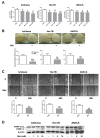
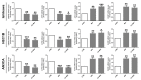
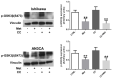
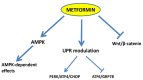
Multiple lines of evidence suggest that metformin, an antidiabetic drug, exerts anti-tumorigenic effects in different types of cancer. Metformin has been reported to affect cancer cells' metabolism and proliferation mainly through the activation of AMP-activated protein kinase (AMPK). Here, we show that metformin inhibits, indeed, endometrial cancer cells' growth and induces apoptosis. More importantly, we report that metformin affects two important pro-survival pathways, such as the Unfolded Protein Response (UPR), following endoplasmic reticulum stress, and the WNT/β-catenin pathway. GRP78, a key protein in the pro-survival arm of the UPR, was indeed downregulated, while GADD153/CHOP, a transcription factor that mediates the pro-apoptotic response of the UPR, was upregulated at both the mRNA and protein level. Furthermore, metformin dramatically inhibited β-catenin mRNA and protein expression. This was paralleled by a reduction in β-catenin transcriptional activity, since metformin inhibited the activity of a TCF/LEF-luciferase promoter. Intriguingly, compound C, a well-known inhibitor of AMPK, was unable to prevent all these effects, suggesting that metformin might inhibit endometrial cancer cells' growth and survival through the modulation of specific branches of the UPR and the inhibition of the Wnt/β-catenin pathway in an AMPK-independent manner. Our findings may provide new insights on the mechanisms of action of metformin and refine the use of this drug in the treatment of endometrial cancer.
Keywords: AMPK; UPR; Wnt/β-catenin; endometrial cancer; metformin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pollak M.N. Investigating metformin for cancer prevention and treatment: The end of the beginning. Cancer Discov. 2012;2:2778–2790. doi: 10.1158/2159-8290.CD-12-0263. - DOI - PubMed
-
- Ezewuiro O., Grushko T.A., Kocherginsky M., Habis M., Hurteau J.A., Mills K.A., Hunn J., Olopade O.I., Fleming G.F., Romero I.L. Association of Metformin Use with Outcomes in Advanced Endometrial Cancer Treated with Chemotherapy. PLoS ONE. 2016;11:e0147145. doi: 10.1371/journal.pone.0147145. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PRIN2017; FSC-progetto "Innovative Devices For SHAping the RIsk of Diabetes" (IDF SHARID)-ARS01_01270/Ministero dell'Istruzione, dell'Università e della Ricerca
- POR FESR 2014-2020-Obiettivo specifico 1.2.-Manifestazione di Interesse per la Realizzazione di Technology Platform nell'ambito della Lotta alle Patologie Oncologiche"-Projects COEPICA, RARE PLAT NET and SATIN/Regione Campania
- grant FLAGSHIP Interomics Project ASPIRE/Consiglio Nazionale delle Ricerche
- 2018-2020/Italian Diabete Ricerca Foundation and Eli Lilly Italy
- European Foundation for the Study of Diabetes (EFSD)/Boehringer Ingelheim (2018-2020)/European Foundation for the Study of Diabetes
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

