CHIR99021 Augmented the Function of Late Endothelial Progenitor Cells by Preventing Replicative Senescence
- PMID: 33946516
- PMCID: PMC8124445
- DOI: 10.3390/ijms22094796
CHIR99021 Augmented the Function of Late Endothelial Progenitor Cells by Preventing Replicative Senescence
Erratum in
-
Correction: Rethineswaran et al. CHIR99021 Augmented the Function of Late Endothelial Progenitor Cells by Preventing Replicative Senescence. Int. J. Mol. Sci. 2021, 22, 4796.Int J Mol Sci. 2022 Oct 27;23(21):13020. doi: 10.3390/ijms232113020. Int J Mol Sci. 2022. PMID: 36362452 Free PMC article.
Abstract
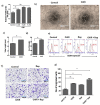
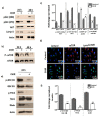
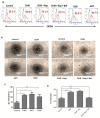
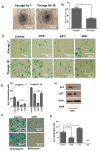
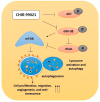
Endothelial progenitor cells (EPCs) are specialized cells in circulating blood, well known for their ability to form new vascular structures. Aging and various ailments such as diabetes, atherosclerosis and cardiovascular disease make EPCs vulnerable to decreasing in number, which affects their migration, proliferation and angiogenesis. Myocardial ischemia is also linked to a reduced number of EPCs and their endothelial functional role, which hinders proper blood circulation to the myocardium. The current study shows that an aminopyrimidine derivative compound (CHIR99021) induces the inhibition of GSK-3β in cultured late EPCs. GSK-3β inhibition subsequently inhibits mTOR by blocking the phosphorylation of TSC2 and lysosomal localization of mTOR. Furthermore, suppression of GSK-3β activity considerably increased lysosomal activation and autophagy. The activation of lysosomes and autophagy by GSK-3β inhibition not only prevented replicative senescence of the late EPCs but also directed their migration, proliferation and angiogenesis. To conclude, our results demonstrate that lysosome activation and autophagy play a crucial role in blocking the replicative senescence of EPCs and in increasing their endothelial function. Thus, the findings provide an insight towards the treatment of ischemia-associated cardiovascular diseases based on the role of late EPCs.
Keywords: CHIR99021; EPC; GSK-3β; autophagy; lysosome; mTOR; senescence.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
-
- Dai X., Yan X., Zeng J., Chen J., Wang Y., Chen J., Li Y., Barati M.T., Wintergerst K.A., Pan K., et al. Elevating CXCR7 Improves Angiogenic Function of EPCs via Akt/GSK-3β/Fyn-Mediated Nrf2 Activation in Diabetic Limb Ischemia. Circ. Res. 2017;120:e7–e23. doi: 10.1161/CIRCRESAHA.117.310619. - DOI - PMC - PubMed
-
- Zhang X.Y., Su C., Cao Z., Xu S.Y., Xia W.H., Xie W.L., Chen L., Yu B.B., Zhang B., Wang Y., et al. CXCR7 upregulation is required for early endothelial progenitor cell-mediated endothelial repair in patients with hypertension. Hypertension. 2014;63:383–389. doi: 10.1161/HYPERTENSIONAHA.113.02273. - DOI - PubMed
-
- Ii M., Takenaka H., Asai J., Ibusuki K., Mizukami Y., Maruyama K., Yoon Y.S., Wecker A., Luedemann C., Eaton E., et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ. Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

