Lung Transplantation: CT Assessment of Chronic Lung Allograft Dysfunction (CLAD)
- PMID: 33946544
- PMCID: PMC8147203
- DOI: 10.3390/diagnostics11050817
Lung Transplantation: CT Assessment of Chronic Lung Allograft Dysfunction (CLAD)
Abstract
Chronic lung allograft rejection remains one of the major causes of morbi-mortality after lung transplantation. The term Chronic Lung Allograft Dysfunction (CLAD) has been proposed to describe the different processes that lead to a significant and persistent deterioration in lung function without identifiable causes. The two main phenotypes of CLAD are Bronchiolitis Obliterans Syndrome (BOS) and Restrictive Allograft Syndrome (RAS), each of them characterized by particular functional and imaging features. These entities can be associated (mixed phenotype) or switched from one to the other. If CLAD remains a clinical diagnosis based on spirometry, computed tomography (CT) scan plays an important role in the diagnosis and follow-up of CLAD patients, to exclude identifiable causes of functional decline when CLAD is first suspected, to detect early abnormalities that can precede the diagnosis of CLAD (particularly RAS), to differentiate between the obstructive and restrictive phenotypes, and to detect exacerbations and evolution from one phenotype to the other. Recognition of early signs of rejection is crucial for better understanding of physiopathologic pathways and optimal management of patients.
Keywords: chronic lung allograft dysfunction; computed tomography (CT) scan; lung transplantation.
Conflict of interest statement
Philippe A. Grenier has received speaker honorarium from Siemens Healthineers.
Figures

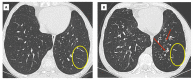
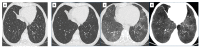
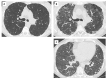
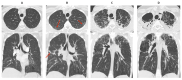

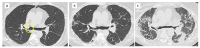
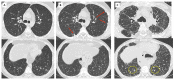
References
-
- Chambers D.C., Cherikh W.S., Goldfarb S.B., Hayes D., Kucheryavaya A.Y., Toll A.E., Khush K.K., Levvey B.J., Meiser B., Rossano J.W., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation Report-2018. J. Heart Lung Transplant. 2018;37:1169–1183. doi: 10.1016/j.healun.2018.07.020. - DOI - PubMed
-
- Sato M., Waddell T.K., Wagnetz U., Roberts H.C., Hwang D.M., Haroon A., Wagnetz D., Chaparro C., Singer L.G., Hutcheon M.A., et al. Restrictive allograft syndrome (RAS): A novel form of chronic lung allograft dysfunction. J. Heart Lung Transplant. 2011;30:735–742. doi: 10.1016/j.healun.2011.01.712. - DOI - PubMed
-
- Verleden G.M., Glanville A.R., Lease E.D., Fisher A.J., Calabrese F., Corris P.A., Ensor C.R., Gottlieb J., Hachem R.R., Lama V., et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment—A consensus report from the pulmonary council of the ISHLT. J. Heart Lung Transplant. 2019;38:493–503. doi: 10.1016/j.healun.2019.03.009. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

