The Role of LGR4 (GPR48) in Normal and Cancer Processes
- PMID: 33946652
- PMCID: PMC8125670
- DOI: 10.3390/ijms22094690
The Role of LGR4 (GPR48) in Normal and Cancer Processes
Abstract
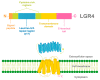
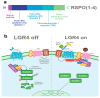
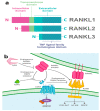
Leucine-rich repeats containing G protein-coupled receptor 4 (LGR4) is a receptor that belongs to the superfamily of G protein-coupled receptors that can be activated by R-spondins (RSPOs), Norrin, circLGR4, and the ligand of the receptor activator of nuclear factor kappa-B (RANKL) ligands to regulate signaling pathways in normal and pathological processes. LGR4 is widely expressed in different tissues where it has multiple functions such as tissue development and maintenance. LGR4 mainly acts through the Wnt/β-catenin pathway to regulate proliferation, survival, and differentiation. In cancer, LGR4 participates in tumor progression, invasion, and metastasis. Furthermore, recent evidence reveals that LGR4 is essential for the regulation of the cancer stem cell population by controlling self-renewal and regulating stem cell properties. This review summarizes the function of LGR4 and its ligands in normal and malignant processes.
Keywords: CSCs; GPR48; LGR4; cancer.
Conflict of interest statement
The authors declare no conflict interest.
Figures
References
-
- Weinberg R.A. The Biology of Cancer. 2nd ed. Garland Science, Taylor and Francis Group; New York, NY, USA: London, UK: 2013.
-
- Krauss G. Biochemistry of Signal Transduction and Regulation. Wiley; Hoboken, NJ, USA: 2003.
-
- Van Loy T., Vandersmissen H.P., Van Hiel M.B., Poels J., Verlinden H., Badisco L., Vassart G., Vanden Broeck J. Comparative genomics of leucine-rich repeats containing G protein-coupled receptors and their ligands. Gen. Comp. Endocrinol. 2008;155:14–21. doi: 10.1016/j.ygcen.2007.06.022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

