Glutathione S-Transferases in Cancer
- PMID: 33946704
- PMCID: PMC8146591
- DOI: 10.3390/antiox10050701
Glutathione S-Transferases in Cancer
Abstract
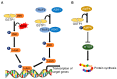
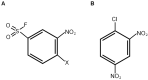
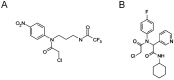
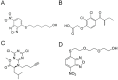
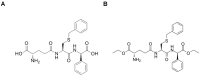
In humans, the glutathione S-transferases (GST) protein family is composed of seven members that present remarkable structural similarity and some degree of overlapping functionalities. GST proteins are crucial antioxidant enzymes that regulate stress-induced signaling pathways. Interestingly, overactive GST proteins are a frequent feature of many human cancers. Recent evidence has revealed that the biology of most GST proteins is complex and multifaceted and that these proteins actively participate in tumorigenic processes such as cell survival, cell proliferation, and drug resistance. Structural and pharmacological studies have identified various GST inhibitors, and these molecules have progressed to clinical trials for the treatment of cancer and other diseases. In this review, we discuss recent findings in GST protein biology and their roles in cancer development, their contribution in chemoresistance, and the development of GST inhibitors for cancer treatment.
Keywords: GST inhibitors; Glutathione S-transferases (GSTs); JNK; antioxidants; apoptosis; cancer-cell signaling; chemoresistance; glutathionylation; metabolism; oxidative stress; patient survival; xenobiotic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kural C., Kocdogan A.K., Şimşek G.G., Oguztuzun S., Kaygın P., Yılmaz I., Bayram T., Izci Y. Glutathione S-transferases and cytochrome P450 enzyme expression in patients with intracranial tumors: Preliminary report of 55 patients. Med. Princ. Pr. 2018;28:56–62. doi: 10.1159/000494496. - DOI - PMC - PubMed
-
- Soleo L., Strzelczyk R. Xenobiotics and glutathione. G Ital. Med. Lav. Ergon. 1999;21:302–308. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

