Toward an Understanding of the Structural and Mechanistic Aspects of Protein-Protein Interactions in 2-Oxoacid Dehydrogenase Complexes
- PMID: 33946784
- PMCID: PMC8146983
- DOI: 10.3390/life11050407
Toward an Understanding of the Structural and Mechanistic Aspects of Protein-Protein Interactions in 2-Oxoacid Dehydrogenase Complexes
Abstract
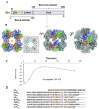
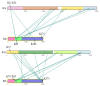
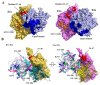
The 2-oxoglutarate dehydrogenase complex (OGDHc) is a key enzyme in the tricarboxylic acid (TCA) cycle and represents one of the major regulators of mitochondrial metabolism through NADH and reactive oxygen species levels. The OGDHc impacts cell metabolic and cell signaling pathways through the coupling of 2-oxoglutarate metabolism to gene transcription related to tumor cell proliferation and aging. DHTKD1 is a gene encoding 2-oxoadipate dehydrogenase (E1a), which functions in the L-lysine degradation pathway. The potentially damaging variants in DHTKD1 have been associated to the (neuro) pathogenesis of several diseases. Evidence was obtained for the formation of a hybrid complex between the OGDHc and E1a, suggesting a potential cross talk between the two metabolic pathways and raising fundamental questions about their assembly. Here we reviewed the recent findings and advances in understanding of protein-protein interactions in OGDHc and 2-oxoadipate dehydrogenase complex (OADHc), an understanding that will create a scaffold to help design approaches to mitigate the effects of diseases associated with dysfunction of the TCA cycle or lysine degradation. A combination of biochemical, biophysical and structural approaches such as chemical cross-linking MS and cryo-EM appears particularly promising to provide vital information for the assembly of 2-oxoacid dehydrogenase complexes, their function and regulation.
Keywords: enzyme catalysis; glucose metabolism; hydrogen exchange mass spectrometry; molecular modeling; neurodegeneration; protein assembly; protein cross-linking; protein-protein interaction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Yap Z.Y., Strucinska K., Matsuzaki S., Lee S., Si Y., Humphries K., Tarnopolsky M.A., Yoon W.H. A biallelic pathogenic variant in the OGDH gene results in a neurological disorder with features of a mitochondrial disease. J. Inherit. Metab. Dis. 2021;44:388–400. doi: 10.1002/jimd.12248. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

