A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer
- PMID: 33946913
- PMCID: PMC8125712
- DOI: 10.3390/cancers13092149
A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer
Abstract
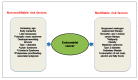
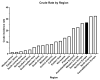
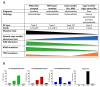
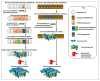
Endometrial cancer is the most common cancer affecting the reproductive organs of women living in higher-income countries. Apart from hormonal influences and genetic predisposition, obesity and metabolic syndrome are increasingly recognised as major factors in endometrial cancer risk, due to changes in lifestyle and diet, whereby high glycaemic index and lipid deposition are prevalent. This is especially true in countries where micronutrients, such as vitamins and minerals are exchanged for high calorific diets and a sedentary lifestyle. In this review, we will survey the currently known lifestyle factors, dietary requirements and hormonal changes that increase an individual's risk for endometrial cancer and discuss their relevance for clinical management. We also examine the evidence that everyday factors and clinical interventions have on reducing that risk, such that informed healthy choices can be made. In this narrative review, we thus summarise the dietary and lifestyle factors that promote and prevent the incidence of endometrial cancer.
Keywords: diabetes; diet; endometrial cancer; hormones; lipids; melatonin; minerals; nutrients; obesity; vitamins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Cancer Research Fund Diet, Nutrition, Physical Activity and Endometrial Cancer. [(accessed on 23 January 2021)]; Available online: https://www.wcrf.org/dietandcancer.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

