Hybrid Drugs-A Strategy for Overcoming Anticancer Drug Resistance?
- PMID: 33946916
- PMCID: PMC8124695
- DOI: 10.3390/molecules26092601
Hybrid Drugs-A Strategy for Overcoming Anticancer Drug Resistance?
Abstract
Despite enormous progress in the treatment of many malignancies, the development of cancer resistance is still an important reason for cancer chemotherapy failure. Increasing knowledge of cancers' molecular complexity and mechanisms of their resistance to anticancer drugs, as well as extensive clinical experience, indicate that an effective fight against cancer requires a multidimensional approach. Multi-target chemotherapy may be achieved using drugs combination, co-delivery of medicines, or designing hybrid drugs. Hybrid drugs simultaneously targeting many points of signaling networks and various structures within a cancer cell have been extensively explored in recent years. The single hybrid agent can modulate multiple targets involved in cancer cell proliferation, possesses a simpler pharmacokinetic profile to reduce the possibility of drug interactions occurrence, and facilitates the process of drug development. Moreover, a single medication is expected to enhance patient compliance due to a less complicated treatment regimen, as well as a diminished number of adverse reactions and toxicity in comparison to a combination of drugs. As a consequence, many efforts have been made to design hybrid molecules of different chemical structures and functions as a means to circumvent drug resistance. The enormous number of studies in this field encouraged us to review the available literature and present selected research results highlighting the possible role of hybrid drugs in overcoming cancer drug resistance.
Keywords: cancer; conjugate; drug resistance; hybrid drug; overcoming anticancer drug resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


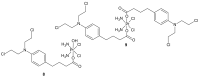


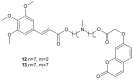


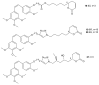

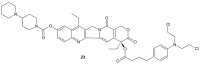

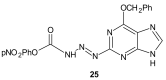
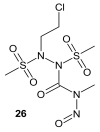
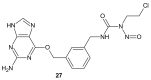

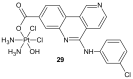
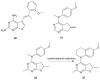

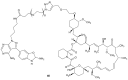

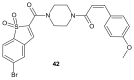
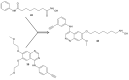

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

