Angiogenic Properties of Concentrated Growth Factors (CGFs): The Role of Soluble Factors and Cellular Components
- PMID: 33946931
- PMCID: PMC8146902
- DOI: 10.3390/pharmaceutics13050635
Angiogenic Properties of Concentrated Growth Factors (CGFs): The Role of Soluble Factors and Cellular Components
Abstract
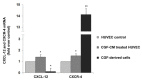
Blood-derived concentrated growth factors (CGFs) represent a novel autologous biomaterial with promising applications in regenerative medicine. Angiogenesis is a key factor in tissue regeneration, but the role played by CGFs in vessel formation is not clear. The purpose of this study was to characterize the angiogenic properties of CGFs by evaluating the effects of its soluble factors and cellular components on the neovascularization in an in vitro model of angiogenesis. CGF clots were cultured for 14 days in cell culture medium; after that, CGF-conditioned medium (CGF-CM) was collected, and soluble factors and cellular components were separated and characterized. CGF-soluble factors, such as growth factors (VEGF and TGF-β1) and matrix metalloproteinases (MMP-2 and -9), were assessed by ELISA. Angiogenic properties of CGF-soluble factors were analyzed by stimulating human cultured endothelial cells with increasing concentrations (1%, 5%, 10%, or 20%) of CGF-CM, and their effect on cell migration and tubule-like formation was assessed by wound healing and Matrigel assay, respectively. The expression of endothelial angiogenic mediators was determined using qRT-PCR and ELISA assays. CGF-derived cells were characterized by immunostaining, qRT-PCR and Matrigel assay. We found that CGF-CM, consisting of essential pro-angiogenic factors, such as VEGF, TGF-β1, MMP-9, and MMP-2, promoted endothelial cell migration; tubule structure formation; and endothelial expression of multiple angiogenic mediators, including growth factors, chemokines, and metalloproteinases. Moreover, we discovered that CGF-derived cells exhibited features such as endothelial progenitor cells, since they expressed the CD34 stem cell marker and endothelial markers and participated in the neo-angiogenic process. In conclusion, our results suggest that CGFs are able to promote endothelial angiogenesis through their soluble and cellular components and that CGFs can be used as a biomaterial for therapeutic vasculogenesis in the field of tissue regeneration.
Keywords: angiogenesis; biomaterials; concentrated growth factors (CGFs); endothelial cells; endothelial markers; endothelial progenitor cells (EPCs); matrix metalloproteinases; pro-angiogenic factors; tissue regeneration; vasculogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Hur J., Yoon C.H., Kim H.S., Choi J.H., Kang H.J., Hwang K.K., Oh B.H., Lee M.M., Park Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. - DOI - PubMed
-
- Asahara T., Masuda H., Takahashi T., Kalka C., Pastore C., Silver M., Kearne M., Magner M., Isner J.M. Bone Marrow Origin of Endothelial Progenitor Cells Responsible for Postnatal Vasculogenesis in Physiological and Pathological Neovascularization. Circ. Res. 1999;85:221–228. doi: 10.1161/01.RES.85.3.221. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

