Cardiac Glycosides as Immune System Modulators
- PMID: 33947098
- PMCID: PMC8146282
- DOI: 10.3390/biom11050659
Cardiac Glycosides as Immune System Modulators
Abstract
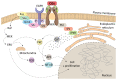
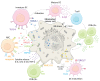
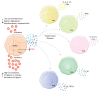
Cardiac glycosides (CGs) are natural steroid compounds occurring both in plants and animals. They are known for long as cardiotonic agents commonly used for various cardiac diseases due to inhibition of Na+/K+-ATPase (NKA) pumping activity and modulating heart muscle contractility. However, recent studies show that the portfolio of diseases potentially treatable with CGs is much broader. Currently, CGs are mostly studied as anticancer agents. Their antiproliferative properties are based on the induction of multiple signaling pathways in an NKA signalosome complex. In addition, they are strongly connected to immunogenic cell death, a complex mechanism of induction of anticancer immune response. Moreover, CGs exert various immunomodulatory effects, the foremost of which are connected with suppressing the activity of T-helper cells or modulating transcription of many immune response genes by inhibiting nuclear factor kappa B. The resulting modulations of cytokine and chemokine levels and changes in immune cell ratios could be potentially useful in treating sundry autoimmune and inflammatory diseases. This review aims to summarize current knowledge in the field of immunomodulatory properties of CGs and emphasize the large area of potential clinical use of these compounds.
Keywords: NKA signalosome; Th17; anticancer compounds; calreticulin; cardiac steroids; immunogenic cell death; inflammation; interleukin 17; retinoic acid receptor-related orphan receptor γ thymus; sodium-potassium ATPase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bejcek J., Spiwok V., Kmonickova E., Ruml T., Rimpelova S. Cardiac glycosides: On their therapeutic potential for cancer treatment. Chem. Listy. 2021;115:4–12.
-
- Garcia I.J.P., de Oliveira G.C., Valadares J.M.D., Banfi F.F., Andrade S.N., Freitas T.R., Filho E.D.S.M., Santos H.D.L., Vieira G.M., Chaves M.H., et al. New bufadienolides extracted from Rhinella Marina inhibit Na,K-ATPase and induce apoptosis by activating caspases 3 and 9 in human breast and ovarian cancer cells. Steroids. 2019;152:108490. doi: 10.1016/j.steroids.2019.108490. - DOI - PubMed
-
- Kanwal N., Rasul A., Hussain G., Anwar H., Shah M.A., Sarfraz I., Riaz A., Batool R., Shahbaz M., Hussain A., et al. Oleandrin: A bioactive phytochemical and potential cancer killer via multiple cellular signaling pathways. Food Chem. Toxicol. 2020;143:111570. doi: 10.1016/j.fct.2020.111570. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

