Dynamics & Spectroscopy with Neutrons-Recent Developments & Emerging Opportunities
- PMID: 33947108
- PMCID: PMC8125526
- DOI: 10.3390/polym13091440
Dynamics & Spectroscopy with Neutrons-Recent Developments & Emerging Opportunities
Abstract
This work provides an up-to-date overview of recent developments in neutron spectroscopic techniques and associated computational tools to interrogate the structural properties and dynamical behavior of complex and disordered materials, with a focus on those of a soft and polymeric nature. These have and continue to pave the way for new scientific opportunities simply thought unthinkable not so long ago, and have particularly benefited from advances in high-resolution, broadband techniques spanning energy transfers from the meV to the eV. Topical areas include the identification and robust assignment of low-energy modes underpinning functionality in soft solids and supramolecular frameworks, or the quantification in the laboratory of hitherto unexplored nuclear quantum effects dictating thermodynamic properties. In addition to novel classes of materials, we also discuss recent discoveries around water and its phase diagram, which continue to surprise us. All throughout, emphasis is placed on linking these ongoing and exciting experimental and computational developments to specific scientific questions in the context of the discovery of new materials for sustainable technologies.
Keywords: computational materials modeling; ice; neutron spectroscopy; nuclear quantum effects; plastic crystals; polymers; soft matter; supramolecular frameworks; sustainable materials; water.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
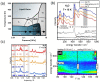













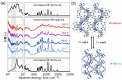

References
-
- Andersen K., Argyriou D., Jackson A., Houston J., Henry P., Deen P., Toft-Petersen R., Beran P., Strobl M., Arnold T., et al. The Instrument Suite of the European Spallation Source. Nucl. Instrum. Methods Phys. Res. 2020;957:163402. doi: 10.1016/j.nima.2020.163402. - DOI
-
- Carpenter J.M. The Development of Compact Neutron Sources. Nat. Rev. Phys. 2019;1:177–179. doi: 10.1038/s42254-019-0024-8. - DOI
-
- Ott F., Menelle A., Alba-Simionesco C. The SONATE Project, a French CANS for Materials Sciences Research. EPJ Web Conf. 2020;231:01004. doi: 10.1051/epjconf/202023101004. - DOI
-
- Otake Y. RIKEN Accelerator-driven Compact Neutron Systems. EPJ Web Conf. 2020;231:01009. doi: 10.1051/epjconf/202023101009. - DOI
-
- Zakalek P., Cronert T., Baggemann J., Doege P.E., Rimmler M., Voigt J., Mauerhofer E., Rücker U., Gutberlet T., Podlech H., et al. High-brilliance Neutron Source Project. J. Phys. Conf. Ser. 2020;1401:012010. doi: 10.1088/1742-6596/1401/1/012010. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

